 |
 |

Mifepristone (RU 486)
Current Knowledge and Future Prospects
Jeffrey R. Goldberg, MD;
Marcus G. Plescia, MD, MPH;
Geraldine D. Anastasio, PharmD
Arch Fam Med. 1998;7:219-222.
ABSTRACT
Mifepristone (RU 486) has received recent attention for its effects as an abortifacient. Mifepristone has not yet been approved for use in the United States. The Food and Drug Administration issued an "approvable letter" in September 1996, but mifepristone will not be available in the United States until a new manufacturer is found. Experience with mifepristone is extensive in Europe, and there have been retrospective studies and large, controlled clinical trials of its efficacy. It is most efficacious when administered to women who are less than 8 weeks pregnant, in a single 600-mg oral dose followed 48 hours later by administration of intravaginal misoprostol. This regimen has a success rate of 98%, as do most surgical abortive procedures. The most frequent adverse effect is painful contractions, which occur in up to 93% of women, with oral analgesia required in as many as half the cases. Large-scale surveys of women who elected medical abortion reported high patient satisfaction. Mifepristone is likely to have additional clinical uses. Researchers are exploring mifepristone's potential uses in cervical ripening and labor induction; contraception; delivery after intrauterine demise; treatment of breast cancer, unresectable meningioma, and prostate cancer; amelioration of endometriosis; and management of Cushing syndrome.
INTRODUCTION
Since its discovery and introduction in the early 1980s, the progesterone and glucocorticoid antagonist mifepristone (RU 486) has been studied for a myriad of clinical conditions. It has also caused great controversy. Although the drug is known (both clinically and politically) for its abortifacient effects, researchers have also studied its effects on cervical ripening, labor induction, delivery of stillborn fetuses, breast cancer, unresectable meningioma, prostate cancer, endometriosis, and Cushing syndrome.1-17 Mifepristone has also been referred to as a contragestational agent because it prevents pregnancy both before and after conception. Until recently, mifepristone was not available for clinical use or research purposes in the United States. However, it has been prescribed for more than a decade in Europe and extensively studied in a large, unblinded, nonrandomized clinical trial in France.1 Its pharmacology, clinical efficacy, and tolerability have been extensively characterized. Despite the highly charged political controversy, recent federal regulatory activity indicates that mifepristone will soon be available for clinical use in the United States.2 This article will provide the practicing physician with a brief overview and summary of the clinical effects of mifepristone and what the future may hold for this compound.
PHARMACODYNAMICS
Mifepristone was originally designed by the French pharmaceutical company Roussel-Uclaf (Romainville, France) as a glucocorticoid antagonist and was only serendipitously found to have antiprogesterone effects. Many of its potential effects are still under active research.3 Mifepristone has 3 primary pharmacological effects: endometrial, gonadotropic, and adrenocortical.
Endometrial Effects
Mifepristone acts as a progesterone antagonist by competing with endogenous progesterone for receptor binding. It binds with very high affinity (2 to 10 times that of progesterone) to these receptors.4 In the absence of progesterone, however, mifepristone can act as a partial agonist.5 The putative molecular mechanism has not been proved. There is evidence that it involves a conformational change in the mifepristone-progesterone receptor complex that renders it inactive and unable to promote transcription of cellular DNA.5 Because progesterone receptors are found primarily in reproductive organs, mifepristone exerts its principal effect on the uterus.6 Mifepristone blocks the effects of natural progesterone on the endometrium and decidua. This leads to degeneration and shedding of the endometrial lining, thereby preventing or disrupting implantation of the conceptus.6 Mifepristone also increases both uterine production of prostaglandins and uterine sensitivity to the contractile effects of prostaglandins.5 It is postulated that mifepristone acts directly on the uterine muscle through an entirely separate mechanism, perhaps by increasing gap junctions in the myometrium.6 Tissue culture studies have shown that mifepristone continues to display procontractile effects on the uterus even when the effects of prostaglandins are neutralized by treatment with indomethacin.4
Gonadotropic Effects
The effects of mifepristone on the hypophysial-ovarian axis have also been studied and reported in the literature. Most of these studies investigated the drug as a contraceptive as opposed to an abortifacient.3 This distinction is important clinically as well as politically. Mifepristone has differing effects on the usual hormonal milieu when it is administered during the menstrual cycle. When given during the follicular phase it is capable of inhibiting folliculogenesis and, subsequently, the normal luteinizing hormone surge for the hypothalamus. This results in an ongoing anovular phase. Safety issues with such a major alteration in normal female hormonal patterns require further investigation.3
Adrenocortical Effects
Mifepristone has antiglucocorticoid effects by binding to glucocorticoid receptors with an affinity that is 2 to 3 times that of dexamethasone. It interferes with cortisol binding to tissue in the hypothalamus. This blocks normal negative feedback mechanisms and causes a compensatory increase in serum levels of both cortisol and corticotropin. In addition, the drug binds to cortisol receptors in the periphery and therefore blocks the effects of circulating cortisol in target tissue.6 Higher doses of mifepristone are needed to produce this antiglucocorticoid effect as opposed to an antiprogestin effect.4-6 Because blockade in the periphery is opposed by increased cortisol and corticotropin secretion, no reports of clinically significant relative cortisol deficiency have been reported when it has been used as an antiprogestin—even with long-term use of mifepristone for several weeks. Mifepristone has almost no affinity for estrogen, androgen, or mineralocorticoid receptors.
PHARMACOKINETICS
Mifepristone has a bioavailability of 70% after oral administration. Peak plasma concentrations are reached in 1 to 2 hours after a single oral dose. It has a half-life of approximately 20 to 30 hours. The pharmacokinetics of mifepristone are nonlinear. Serum drug concentrations increase progressively after oral doses from 50 to 100 mg, but no further increases occur after doses of 100 to 800 mg. This finding is partly explained by the progressive saturation of  1-acid glycoprotein, the serum binding protein for mifepristone. The unbound mifepristone is quickly metabolized in the liver by a 2-step process, demethylation and hydroxylation, with metabolites detectable in plasma about 1 hour after oral ingestion.7 The concentration of metabolites increases in a dose-dependent manner. Metabolites bind to progesterone receptors with an affinity of 10% to 20% that of the parent compound. These metabolites probably contribute little to the pharmacologic effect of mifepristone. Both mifepristone and its metabolites are excreted primarily in the feces via the biliary system. Little is cleared by the kidneys.4-5,8 Mifepristone crosses the placenta. The maternal-fetal ratio in plasma is approximately 9:1.4 1-acid glycoprotein, the serum binding protein for mifepristone. The unbound mifepristone is quickly metabolized in the liver by a 2-step process, demethylation and hydroxylation, with metabolites detectable in plasma about 1 hour after oral ingestion.7 The concentration of metabolites increases in a dose-dependent manner. Metabolites bind to progesterone receptors with an affinity of 10% to 20% that of the parent compound. These metabolites probably contribute little to the pharmacologic effect of mifepristone. Both mifepristone and its metabolites are excreted primarily in the feces via the biliary system. Little is cleared by the kidneys.4-5,8 Mifepristone crosses the placenta. The maternal-fetal ratio in plasma is approximately 9:1.4
USE IN PREGNANCY TERMINATION
Most research and clinical experience with mifepristone involves its use as an abortifacient. Initial pilot studies and subsequent clinical trials have been done primarily by investigators in France. Early studies investigated the use of mifepristone (then known as RU 486) alone for the termination of early first-trimester pregnancies.4, 6, 8 Success was defined as complete expulsion of the conceptus without the need for any "rescue"-type surgical procedure. All other outcomes (ongoing pregnancy, incomplete abortion, or the necessity for a hemostatic surgical procedure) were considered failures. These early studies used mifepristone in various doses over variable durations (50-800 mg over 1 to 7 days) in women with amenorrhea for less than 9 weeks. Results were variable but showed clinical success in only 50% to 85%. Success rates were lower in women with high quantitative 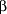 -human chorionic gonadotropin levels, ie, those who had been pregnant longer. In fact, those with -human chorionic gonadotropin levels, ie, those who had been pregnant longer. In fact, those with 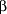 -human chorionic gonadotropin levels greater than 19800 IU/L were 2.8 times as likely to have treatment fail as those with -human chorionic gonadotropin levels greater than 19800 IU/L were 2.8 times as likely to have treatment fail as those with 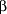 -human chorionic gonadotropin levels less than 6358 IU/L.8 It was subsequently shown in larger studies and by retrospective observation that the maximal success rate was achieved with a single dose of 600 mg of mifepristone administered to women who had been amenorrheic for less than 42 days (which is 2 weeks of missed menses).4, 8-9 -human chorionic gonadotropin levels less than 6358 IU/L.8 It was subsequently shown in larger studies and by retrospective observation that the maximal success rate was achieved with a single dose of 600 mg of mifepristone administered to women who had been amenorrheic for less than 42 days (which is 2 weeks of missed menses).4, 8-9
Prostaglandins play a major role in stimulating uterine contraction. Results of tissue studies have shown increased sensitivity of the uterus to prostaglandins when they are administered with mifepristone. Endogenous prostaglandins cause regular uterine contractions beginning from 24 to 36 hours after administration of mifepristone. These findings led to the development of sequential administration regimens of mifepristone and a low dose of prostaglandin analogue administered 36 to 72 hours later.8 Studies using differing types of prostaglandins, administered orally, vaginally, or by the intramuscular route, showed clinical success rates of 96% or greater in women with amenorrhea for 49 days or less.6, 10-11 One study proved that vaginal administration of misoprostol (Cytotec, a synthetic prostaglandin E1 analogue; G. D. Searle & Company, Skokie, Ill) was more effective and better tolerated than oral administration.12
The most recently published study is a prospective clinical trial of 166 subjects in the United States. A regimen of oral mifepristone, 600 mg, followed 48 hours later by home administration of misoprostol, 800 mg (as four 200-mg tablets) intravaginally, was evaluated for pregnancy termination at up to 8 weeks of gestation (56 days or less by transvaginal sonogram).13 Of the subjects, 82% were white, their mean age was 27 years, and the mean gestational age was 6 weeks 1 day. If the gestational sac was still present on ultrasound at 7 days, a repeated dose of misoprostol was administered. Complete abortion occurred in 98% of the subjects. Only 4% of subjects required a second dose of misoprostol.
SIDE EFFECTS AND ADVERSE OUTCOMES
The most frequent adverse effect is abdominal pain during the 4-hour period after administration of the prostaglandin analogue. This is reported in up to 93% of women and has been shown to increase proportionally with increasing prostaglandin dose.1 Pain generally responds to oral analgesia and is significantly less than for abortive attempts using prostaglandins alone.1, 4 In the most recent study, one third of the 166 subjects reported nausea with both medications and 68% used an oral analgesic. Other side effects include vomiting (19%), diarrhea (22%), cramping (91%), dizziness (37%), headache (19%), and fever, warmth, or chills (37%). However, 96% of the subjects agreed that the procedure "went well," and 90% agreed that home administration of misoprostol was acceptable.13
Serious bleeding requiring transfusion occurs in less than 1% of patients. This rate is equal to or lower than that cited for most large series of abortions by vacuum technique but underscores the need to provide ready access to after-hours care and close follow-up.6
It is recommended that intrauterine pregnancy be confirmed before use of mifepristone, since it is not known to be an effective treatment of ectopic pregnancy. There have been no reports of teratogenicity in humans treated with mifepristone. However, those women in whom therapy fails should undergo surgical abortion because of concerns about fetal malformations in animals.
OTHER CLINICAL USES
Postcoital Contraception/Contragestation
Currently there are several highly effective methods in prevention of pregnancy after a single episode of unprotected intercourse. These include both high-dose estrogen alone and estrogen-progestogen combinations, sometimes referred to as "the morning-after pill." These treatments are effective only before implantation of the conceptus and are most effective within 72 hours of coitus. Mifepristone is effective regardless of implantation and can be administered up to 12 to 17 days after intercourse.6 In repeated studies, a single 600-mg dose of mifepristone alone has been shown to be 94% to 100% effective for preventing pregnancy when administered almost anytime before expected date of menses.5, 10 Two studies compared the effectiveness of mifepristone with that of other treatments as a postcoital contraceptive (Table 1). Mifepristone was as effective as the other treatments and produced fewer side effects.14-15
These findings suggest that a regimen of monthly mifepristone could be used as a regular contraceptive treatment. However, monthly administration of mifepristone often alters the timing of the subsequent month's cycle, making its use difficult and impractical as a monthly birth-control device.6, 16
Cervical Ripening
Mifepristone, as a single 600-mg dose, causes softening and dilation of the cervix. Studies have shown that the drug reduces the amount of objective and subjective force necessary to dilate the cervix in preparation for first-trimester surgical abortions without the mechanical problems of laminaria tents or the side effects and medical contraindications of prostaglandins.5-6 Cervical softening has been shown to decrease the morbidity of the procedure. In several studies of second-trimester pregnancy termination, mifepristone administration has been shown to drastically decrease the time from prostaglandin administration to expulsion of the conceptus.4 At this time, however, there are only preliminary studies, and more data are needed before this use can be recommended.
Intrauterine Fetal Death/Nonviable Early Pregnancy
Several studies have shown that mifepristone treatment can induce labor faster than placebo in cases of intrauterine fetal death. In addition, mifepristone may be useful in the treatment of early pregnancy failures associated with in vitro fertilization and artificial insemination or implantation techniques used for infertile couples. Nonviable pregnancies pose a risk of coagulopathy if the pregnancy remains in the uterus for more than 4 weeks. In 1 preliminary study, a single 600-mg dose of mifepristone resulted in uterine evacuation in 100% of patients with failed embryo transfer.4-6
Labor Induction
In a randomized, double-blind study on the proposed use of mifepristone, 200 mg daily for 2 days, for labor induction in 62 postdate pregnancies, 18 (58%) of 31 treated with mifepristone compared with 7 (23%) of 31 who received placebo went into spontaneous labor. The interval to the start of labor was shortened, the need for prostaglandin use was reduced, and the amount of oxytocin needed decreased. Questions about untoward effects on the fetus need to be resolved before this treatment can be recommended on a large scale.4-5
Unresectable Meningioma
Meningiomas have large concentrations of progesterone receptors. As with breast cancer, which has been shown to be responsive to antiestrogens, patients with unresectable meningiomas have been treated with mifepristone for long periods. In 1 small series of 13 patients, treatment with 200 mg of mifepristone daily resulted in minor tumor regression in 5 patients and stabilization in an additional 5.4-6 Several other small series have also shown promise, and there is ongoing research in this area.
Endometriosis
It is known that there are progesterone receptors on endometrial implants in women with endometriosis. Small, uncontrolled trials have shown that mifepristone can decrease pain in women with diagnosed endometriosis. Yet, there was no objective decrease in the extent of ectopic endometrial implants on follow-up laparoscopy.5
Cushing Syndrome
The fact that mifepristone is a glucocorticoid receptor antagonist makes it a plausible drug for treating inoperable Cushing syndrome caused by ectopic corticotropin secretion or adrenocortical carcinomas. Mifepristone binds to cortisol receptors and blocks the effect of excess cortisol in the circulation. Larger doses of mifepristone must be used to obtain the antiglucocorticoid effect. Typical doses have ranged from 5 to 22 mg/kg. In a few case studies, various peripheral markers of relative hypercortisolism have improved after long-term treatment with mifepristone.17
CONCLUSION
No discussion of mifepristone would be complete without allusion to the intense political, ethical, and moral controversy that it has engendered. Many scientific authorities agree with Weiss6 when he states: "If RU 486 were not an abortifacient, its other potential uses would clearly make it an important new drug, worthy of clinical investigation and possible introduction into the American pharmacopeia."6 The discussion on either side of the issue, as with all controversy surrounding abortion, is highly charged and emotional. Mifepristone has not yet been approved for use in the United States; it will not be available in the United States until a new manufacturer is found. Mifepristone is safe for the patient and an effective abortifacient. We as clinicians must be informed about this drug—regardless of our personal views.
AUTHOR INFORMATION
Accepted for publication July 14, 1997.
Reprints: Geraldine D. Anastasio, PharmD, Department of Family Practice, Carolinas Medical Center, Charlotte, NC 28232-2861.
From the Department of Family Practice, Carolinas Medical Center, Charlotte, NC (Drs Goldberg, Plescia, and Anastasio); Charlotte Area Health Education Center (Drs Plescia and Anastasio); and School of Pharmacy (Dr Anastasio) and Department of Family Medicine, School of Medicine (Drs Plescia and Anastasio), University of North Carolina, Chapel Hill. Dr Goldberg is now with Pro Health Physicians, Family Medical Group, Bristol, Conn.
REFERENCES
1. Silvestre L, Dubois C, Renault M, et al. Voluntary interruption of pregnancy with mifepristone (RU 486) and a prostaglandin analogue: a large scale French experience. N Engl J Med. 1990;322:645-648.
WEB OF SCIENCE
| PUBMED
2. The fight for mifepristone (RU 486). Available at: http://www.naral.org/publications/facts/ruchrono.html. Accessed November 6, 1996.
3. Woolley RJ. Contraception—a look forward, II: mifepristone and gossypol. J Am Board Fam Pract. 1991;4:103-113.
4. Brogden RN, Goa KL, Faulds D. Mifepristone: a review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential. Drugs. 1993;45:384-409.
WEB OF SCIENCE
| PUBMED
5. Spitz IM, Bardin CW. Mifepristone (RU 486): a modulator of progestin and glucocorticoid action. N Engl J Med. 1993;329:404-412.
FULL TEXT
|
WEB OF SCIENCE
| PUBMED
6. Weiss BD. RU 486: the progesterone antagonist. Arch Fam Med. 1993;2:63-70.
FREE FULL TEXT
7. Heikinheimo O, Kekkonen R. Dose-response relationship of RU 486. Ann Med. 1993;25:71-76.
WEB OF SCIENCE
| PUBMED
8. Avrech OM, Golan A, Weinraub Z, Bukovsky I, Caspi E. Mifepristone (RU 486) alone or in combination with a prostaglandin analogue for termination of early pregnancy: a review. Fertil Steril. 1991;56:385-393.
WEB OF SCIENCE
| PUBMED
9. Couzinet B, LeStrat N, Ulmann A, Baulieu EE, Schaison G. Termination of early pregnancy by the progesterone antagonist RU 486 (mifepristone). N Engl J Med. 1986;315:1565-1570.
WEB OF SCIENCE
| PUBMED
10. Peyron R, Aubeny E, Tarzus V, et al. Early termination of pregnancy with mifepristone (RU 486) and orally active prostaglandin misoprostol. N Engl J Med. 1993;328:1509-1513.
FULL TEXT
|
WEB OF SCIENCE
| PUBMED
11. Thong KJ, Baird DT. Induction of abortion with mifepristone and misoprostol in early pregnancy. Br J Obstet Gynaecol. 1992;99:1004-1007.
WEB OF SCIENCE
| PUBMED
12. El-Rafaey H, Rajasekar D, Abdalla M, Calder L, Templeton A. Induction of abortion with mifepristone (RU 486) and oral or vaginal misoprostol. N Engl J Med. 1995;332:983-987.
FULL TEXT
|
WEB OF SCIENCE
| PUBMED
13. Schaff EA, Stadalius LS, Eisinger SH, Franks P. Vaginal misoprostol administered at home after mifepristone (RU486) for abortion. J Fam Pract. 1997;44:353-360.
WEB OF SCIENCE
| PUBMED
14. Glasier A, Thong KJ, Dewar M, Mackie M, Baird DT. Mifepristone (RU486) compared with high-dose estrogen and progestogen for emergency postcoital contraception. N Engl J Med. 1992;327:1041-1044.
WEB OF SCIENCE
| PUBMED
15. Webb AMC, Russell J, Elstein M. Comparison of Yuzpe regimen, danazol, and mifepristone (RU486) in oral postcoital contraception. BMJ. 1992;305:927-931.
16. Haspels AA. Emergency contraception: a review. Contraception. 1994;50:101-108.
FULL TEXT
|
WEB OF SCIENCE
| PUBMED
17. Nieman LK, Chrousos GP, Kellner C, et al. Successful treatment of Cushing's syndrome with the glucocorticoid antagonist RU 486. J Clin Endocrinol Metab. 1985;61:536-540.
FREE FULL TEXT
THIS ARTICLE HAS BEEN CITED BY OTHER ARTICLES
Mifepristone (RU 486) in Cushing's syndrome
Johanssen and Allolio
Eur J Endocrinol 2007;157:561-569.
ABSTRACT
| FULL TEXT
Mifepristone-induced Early Abortion and Outcome of Subsequent Wanted Pregnancy
Chen et al.
Am J Epidemiol 2004;160:110-117.
ABSTRACT
| FULL TEXT
The Human Orphan Receptor PXR Messenger RNA Is Expressed in Both Normal and Neoplastic Breast Tissue
Dotzlaw et al.
Clin. Cancer Res. 1999;5:2103-2107.
ABSTRACT
| FULL TEXT
|