 |
 |

Prescribe for Health
Improving Cancer Screening in Physician Practices Serving Low-Income and Minority Populations
Clara Manfredi, PhD;
Ronald Czaja, PhD;
Sally Freels, PhD;
Mitchell Trubitt, MD;
Richard Warnecke, PhD;
Loretta Lacey, DrPH, RN
Arch Fam Med. 1998;7:329-337.
ABSTRACT
Objective To evaluate a health maintenance organization (HMO)–sponsored intervention to improve cancer screening in private physician practices serving low-income, minority populations.
Design A randomized controlled trial with preintervention and postintervention measurements. Measurements were obtained by abstracting information from independent random samples of medical charts (N=2316 at preintervention and 2238 at postintervention).
Setting Forty-seven primary care physician practices located in low-income and minority urban neighborhoods in Chicago, Ill.
Intervention Practices were encouraged to adopt an office chart reminder system and to use a patient health maintenance card. Activities to facilitate the adoption of these items and for compliance with cancer screening guidelines included on-site training and start-up assistance visits, a physician continuing medical education seminar, and quality assurance visits with feedback to physicians.
Main Outcome Measures The proportions of patients with a chart-documented mammogram, clinical breast examination, Papanicolaou smear, or fecal occult blood slide test in the 2 years before preintervention and postintervention chart abstractions.
Results Between baseline and postintervention, there was a net increase in the proportion of HMO members in the intervention, compared with the control practices, who received in the preceding 2 years a Papanicolaou smear (11.9%) and a fecal occult blood slide test (14.1%). There was a net increase in the proportion of non-HMO patients in the intervention compared with the control practices who received a clinical breast examination (15.3%) and a fecal occult blood slide test (20.2%).
Conclusions Implementation of an HMO-mediated, multicomponent intervention to improve cancer screening was feasible and effective for the Papanicolaou smear, fecal occult blood slide test, and the clinical breast examination, but not for mammography.
INTRODUCTION
IN 1991, the National Cancer Institute (NCI) launched a research effort designated Prescribe for Health. Several studies were funded to develop and evaluate strategies to increase the application of cancer screening guidelines, especially to underserved populations. The data presented in this article are from one Prescribe for Health study that focused on primary care physicians serving low-income, urban minority populations.
The intervention promoted the regular application of the clinical breast examination (CBE) and mammography for early detection of breast cancer, the Papanicolaou smear for cervical cancer, and the fecal occult blood slide test for colorectal cancer. Improved screening and early detection could substantially reduce mortality from these cancers. Their 5-year survival rate is about 90% when detected while still localized, but only slightly more than 50% of breast and cervical cancers and about one third of colorectal cancers are diagnosed while localized. These proportions are even lower for African Americans.1 The likelihood of receiving cancer detection procedures decreases with older age and with minority and low socioeconomic status.2-4
Providers appear to be aware of, and interested in, cancer detection procedures.5-6 Guidelines for the application of such procedures have been issued by the American Cancer Society and the NCI.7-8 Still, the procedures are inadequately applied in physician practices.5, 9 Physicians report the following barriers to implementing screening guidelines: time constraints, competing health problems, inadequate expertise, insufficient space to carry out the procedures, inadequate reimbursement, provider forgetfulness and inconvenience, and office organization factors.8, 10-11 Patient reluctance to undergo a recommended examination or test may also account for poor screening rates. Perhaps patients do not perceive the importance of the tests, they may be unwilling to undergo a painful or uncomfortable procedure, the cost may be prohibitive, or they may be afraid of treatment or death should a cancer be detected.5, 10-11 However, lack of physician recommendation is a major patient-reported reason for never having had a mammogram.12 Providers' perceptions of their patients may also influence who will or will not receive cancer early detection procedures and information.9, 13 African Americans are less likely than whites to receive cancer early-detection tests or other preventive health care measures even when they receive regular medical checkups,14 are in similar health care settings,15 or are using Medicare.16
Studies of preventive care in disadvantaged populations have tended to focus on public sector medical facilities. Little is known about physicians in the private sector who provide care to large patient populations in communities that are predominantly poor and minority. The practices of these physicians, compared with those of private physicians serving general population patients, tend to be characterized by a disproportionate amount of illness care, more unscheduled visits, and a substantial proportion of patients whose care is not reimbursed through third-party payers. Many of the above barriers are likely to be more pronounced in these settings. As a result, private practices serving poor or minority patients may offer less than optimal opportunities for performing screening procedures and present considerable challenges for primary care providers.
Several studies have tested strategies to increase physician performance of preventive care procedures. Effective interventions have included office reminder systems, medical record aids that highlight needed procedures, audits with feedback on performance, physician continuing education, and patient education.5, 17-20 Comprehensive reviews have concluded that interventions to improve preventive care are most effective when they include several of these components. Multicomponent interventions are most effective when they (1) facilitate the identification of patients in need of services through customized patient intake forms, computerized reminders, etc; (2) facilitate tracking of services over time through flow sheets, patient minirecords, etc; (3) reinforce positive patient behavior through discussions of patient-held health cards, notification and discussion of negative screening results, etc; and (4) reinforce provider behavior with feedback based on chart audits.5, 11, 21-22 For several of these components, successful implementation is more likely if office staff are involved in the process.23-24
Evidence of the effectiveness of these intervention strategies comes primarily from research conducted in academic or hospital settings under controlled conditions that are unlikely to exist in the average community physician's office. These strategies need to be tested in community-based, private physician practices as part of ongoing daily routines.5, 11 To have a significant impact at the population level, effective intervention strategies must be applied on a large scale, through medical systems or institutions that providers respect, and through established mechanisms that can maintain the intervention over time. The Prescribe for Health initiative recognized these issues and required that (1) the interventions be delivered through intermediary medical institutions reaching large populations and (2) the interventions could easily be maintained by the intermediary if proven effective.
METHODS
THE STUDY MEDICAL PRACTICES
The study was conducted in cooperation with a health maintenance organization, Chicago HMO (CHMO). Fifty-two practices that accepted members of CHMO were identified by the CHMO medical director by their address as being located in primarily black and Hispanic low–socioeconomic status neighborhoods in Chicago, Ill. Of these, 51 agreed to participate in the study. Between preintervention and postintervention time points, 3 practices closed, 1 switched to primarily pediatric patients, 1 no longer accepted CHMO patients, and 1 split into 2 separate practices (the preintervention data from the original practice were used as reference for both of these practices). At postintervention there were 47 practices: 24 in the intervention and 23 in the control condition.
The 1990 census data for the tracts in which these practices were located confirmed that they served the target population. Thirty-nine practices were located in census tracts with populations that were 75% to 99% black and/or Hispanic. One third were in census tracts with more than 25% of families with income below poverty level. Across all census tracts, the mean percentage of female-headed households was 37.2%, the mean of median income was $26239, the mean percentage of persons unemployed was 24.7%, and the mean percentage who had moved in the last 5 years was 45.2%. Patient information about education, income, or ethnicity was missing in about half of the patient medical charts and thus was not usable as study variables.
THE INTERVENTION
The intervention promoted the application of the 1987 NCI Working Guidelines for screening for breast, cervical, and colorectal cancer. The guidelines included the following recommendations for providers.8 For breast cancer screening, all women should have CBEs performed by their physicians at least annually. Women between the ages of 40 and 49 years should have a mammogram once every 2 years, and those aged 50 years and older should have a yearly mammogram. For cervical cancer screening, all women who have reached the age of 18 years or who are or have been sexually active should have a yearly Papanicolaou smear and pelvic examination. After a woman has had 3 or more consecutive negative annual Papanicolaou smears, the test may be performed less frequently at her physician's discretion. Beginning at age 50 years, men and women should receive annual fecal occult blood slide testing for early detection of colorectal cancer.
Components of the intervention program are summarized in Table 1. The main components intended to become part of routine procedures were the office chart reminder system, the patient health maintenance card (PHMC), and the changes in the CHMO's quality assurance (QA) protocols that would reinforce program maintenance over time. The reminder system consisted of a chart flow sheet to facilitate recording and tracking of cancer detection tests and a chart sticker to alert physicians that a patient was due for a test. The PHMC replicated the flow sheet information for the patients and was intended to increase demand for, and facilitate, discussion of cancer-detection procedures with physicians or nurses.
|
|

|
|
Table 1. Components of the Prescribe for Health Intervention*
|
|
|
The intervention started in September 1992 with a letter from CHMO to the administrators and medical directors at all the study sites. The letter recommended adoption of the NCI guidelines and provided a supply of the intervention flow sheets with written instructions for their application. Chicago HMO also mailed the PHMC to all its members enrolled at the 24 intervention practices and provided additional cards to the intervention practices.
Training sessions were conducted on site at each intervention practice by research staff. The training focused on the NCI screening guidelines and on how to use the chart reminder system and discuss the PHMC with patients. All relevant support personnel received training, either at the initial session or through additional contacts. Two follow-up assistance visits were made to each intervention practice about 3 and 6 weeks after the training, to reinforce the initial training and to answer potential staff questions.
The 87 primary care physicians from the intervention practices were invited to a continuing medical education (CME) seminar on early cancer detection and management. The seminar was sponsored jointly by the University of Illinois College of Medicine and School of Public Health, Chicago, and CHMO, and had speakers who were well known in the local medical community. Attendance qualified physicians for 3 CME credits. Evaluation of the seminar by those attending was very positive, but only 16 (18%) of the invited physicians attended. Twelve nonphysician practice personnel also attended.
The intervention maintenance strategies were incorporated into the existing CHMO QA system and included periodic chart reviews and feedback to the practices on their performance. The QA forms and protocols were revised to incorporate the cancer screening guidelines. Two rounds of QA visits were conducted about 6 and 12 months after the initial training, which focused on cancer screening. Feedback to the intervention practices included a Cancer Screening Report, which contained findings from the QA chart audits and a summary of the overall and the specific practice's cancer screening rates found in the study baseline chart abstractions.
STUDY HYPOTHESES
The main study assumption was that the CHMO-mediated intervention would improve cancer screening in the population of CHMO patients seen in these practices. Therefore, patients were the unit of analysis. No assumptions were made about either practice-level or individual provider-level intervention effects. Specifically, for each targeted examination (CBE, mammography, Papanicolaou smear, and fecal occult blood slide), hypothesis 1 was that the increase in the proportion of eligible CHMO patients screened between baseline and postintervention would be larger in the intervention practices than in the control practices.
A second assumption was that the intervention would increase physicians' attention to early cancer detection and its effect would diffuse to other, non-CHMO patients. Thus, for each examination we tested hypthesis 2, which stated that an increase in the proportion of non-CHMO patients screened between baseline and postintervention would be larger in the intervention practices than the control practices.
EVALUATION DESIGN
The evaluation of the intervention used a 2-group experimental design with matched pairs and random assignment of medical practices to the control or intervention condition. The main study outcomes were the proportions of eligible patients who received the targeted cancer detection procedures during a 2-year period. Outcomes were assessed through medical chart reviews conducted in 1991, covering 2 years before the intervention, and in 1994, covering the 2 years since the beginning of the intervention. Independent random samples of patient medical charts were selected at baseline and at postintervention.
The participating medical practices were randomly assigned to the intervention or control condition by means of the following 3 matching factors: (1) number of primary care providers (family or general practice, internal medicine, obstetrics/gynecology) at the practice, (2) whether the practice was located in a predominantly black or Hispanic neighborhood, and (3) a composite measure of the estimated proportion of eligible patients at each practice who had received screening procedures consistent with the NCI guidelines for the 4 screening procedures. This last measure was compiled from baseline chart data. Intervention practices received all the intervention components described in Table 1. Control practices received only the CHMO letter announcing the new emphasis on cancer control with a supply of flow sheets. Control practices did not receive the staff training, the start-up assistance visits, or the feedback materials with information from the QA visits, their physicians were not invited to the CME seminar, and the Health Promotion Card was not mailed to their patients.
PATIENT MEDICAL CHART SAMPLING
All 47 practices allowed baseline and postintervention abstraction of the charts of the CHMO patients. Five intervention and 7 control sites refused access to the records of non-CHMO patients at baseline and/or postintervention. Random samples of 60 charts per practice were selected: approximately 20 charts of CHMO patients aged 18 to 39 years, 20 charts of CHMO patients aged 40 years or older, and 20 charts of non-CHMO patients aged 18 years or older. The CHMO patients were randomly selected from the CHMO membership roster for each practice. Because no rosters were available for the non-CHMO patients, a different sampling method was used for them. Ruler measurements were taken of chart storage space, and a pilot sample was selected to estimate the proportion of eligible charts. Then, a systematic random sample of chart inches was selected. The third chart after each random interval was selected for evaluation. This procedure resulted in a range of 19 to 27 eligible charts for non-CHMO patients at the cooperating sites. To ensure that an opportunity for screening had occurred, CHMO and non-CHMO patients qualified for abstraction only if they had been seen at the clinic by a primary health care physician or nurse practitioner at least once during the year before the date of abstraction. To ensure a minimum of continuity of care, patients qualified only if they also had at least 1 visit more than 1 year before the date of abstraction. Finally, the data presented in this article include only cases whose first visit to the clinic was at least 2 years before the chart abstraction date. The eligible sample from the 47 practices included 2316 charts at baseline and 2238 at postintervention.
Not every case in the study was eligible for all cancer early-detection procedures. The screening rates for each procedure were calculated on the number of cases eligible for that procedure. All women aged 18 years or older were eligible for CBEs and for Papanicolaou smears if they had not had a full hysterectomy. All women aged 40 years or older were eligible for mammography (reflecting the NCI and American Cancer Society screening guidelines at that time6). All women and men aged 50 years or older were eligible for fecal occult blood slide tests.
Because the practices varied in size, each case in the final data set was weighted by the reciprocal of its probability of selection. For each of the 47 practices, weights were calculated separately for the 2 CHMO subsamples (younger than 40 years and 40 years or older) and the non-CHMO samples. Weights for both waves were calculated on the basis of the probabilities of selection in the baseline sampling. The results presented include both the weighted population estimates and the unweighted sample sizes.
Although random assignment procedures ensured comparability of study groups on practices' characteristics, group comparability might be affected if they had different compositions of patient characteristics. However, both the baseline and postintervention samples and the intervention and control group were similar on patient sex, age, type of insurance, and, overall, in continuity of care at the same practice.
CHART ABSTRACTION PROCEDURES
Chart abstractions and on-site sampling of the non-CHMO charts were performed by specially trained abstractors from the Survey Research Laboratory of the University of Illinois at Chicago. Sampling and chart abstraction procedures were the same at both preintervention and postintervention waves. Strict protocols and supervisory procedures were followed to ensure data quality. Protocols for chart abstraction required a thorough reading of each chart and coding multiple pieces of information for each patient visit. Ten percent of each abstractor's work was reviewed by the study supervisor and, if unsatisfactory, all the work from that individual was reabstracted by the supervisor. A provider's notation in the medical chart that CBE was performed was considered acceptable evidence of the procedure. For mammography, Papanicolaou smears, and fecal occult blood slides, evidence of the test having occurred consisted of either the laboratory report being present in the medical chart or notations from the provider clearly stating that the test was done and recording its results. At postintervention, information from the intervention flow sheet was not used as evidence that a cancer detection procedure had been performed. The protocol was to examine thoroughly each patient chart and find the required information.
DATA ANALYSIS
SEs of Estimated Screening Rates
The SE of each estimated screening rate is a function of the variances of rate estimates within each practice. The variance of the rate estimate for any given practice is a function of the actual number of eligible cases observed in that practice, as well as the sample weights associated with each eligible case. A practice with a small number of eligible patients and large sample weights will appropriately have a large error variance associated with the rate estimate. Our conclusions about the overall population are then based on estimates and SEs that account for the total error variance across all practices.
Intervention Outcomes
To test the 2 study hypotheses regarding the effectiveness of the intervention, interaction terms were computed as the difference between the average change from baseline to postintervention in the intervention practices and the average change across time in the control practices. Each interaction term was tested for significance against zero by dividing the interaction term by its SE and comparing with a standard normal distribution. Standard errors were computed by means of standard variance estimates for each screening rate, which also incorporated the sampling weights.
Power Calculations
We computed the power of our sample to detect each interaction based on our observed variance estimates for each interaction term. We computed the power to detect an interaction of 15%. We considered 15% to be a reasonable estimate of an important interaction; the interactions that were detected were of approximately this magnitude or stronger. We estimate that our sample provided us with the following probabilities of detecting a significant result, given that the true interaction in the population is 15%: (1) in CHMO patients, 90% for CBEs, 61% for mammography, 86% for Papanicolaou smears, and 89% for fecal occult blood slides, and (2) in non-CHMO patients, 57% for CBEs, 43% for mammography, 56% for Papanicolaou smears, and 74% for fecal occult blood slides.
RESULTS
Table 2 presents patient characteristics from the samples at baseline and at postintervention by study group. There were no major differences in the samples in sex, age, type of insurance, number of visits, or continuity of care.
|
|

|
|
Table 2. Characteristics of Patients in the Study Sample at Baseline and After Intervention by Study Group*
|
|
|
Hypothesis 1 postulated that any increase between baseline and postintervention in the proportion of eligible CHMO patients screened would be significantly larger in the intervention practices than in the control practices. Table 3 shows that the hypothesis was supported for Papanicolaou smears and fecal occult blood slide tests but not for CBEs and mammography. The proportions of patients in the intervention practices receiving a Papanicolaou smear in the preceding 2 years increased from 55.7% at baseline to only 59.7% at postintervention. However, in the control group, the proportion with a Papanicolaou smear decreased from 56.1% at baseline to 48.2% at postintervention, producing a significant net difference between the 2 study groups of 11.9%. A similar pattern occurred for the use of fecal occult blood slides. In the intervention practices, the proportion of eligible patients with at least 1 fecal occult blood slide in the previous 2 years increased from 3.2% at baseline to 12.5% at postintervention, while it decreased from 9.2% to 4.4% in the control practices, a net difference of 14.1%. These interactions were significant at P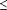 .05. .05.
|
|

|
|
Table 3. Percentages of Patients Who Had Each Examination in the 2 Years Before Baseline and Postintervention Chart Review by Study Group
|
|
|
The proportion of intervention patients with a CBE in the last 2 years increased by 7.7%, from 34.8% at baseline to 42.5% at postintervention. However, a similar increase also occurred in the control practices. The proportion of intervention patients with at least 1 mammogram in the previous 2 years decreased from 38.5% at baseline to 24.6% at postintervention. There was also a small decrease in the control practices.
Hypothesis 2 postulated that the effect of the CHMO intervention would diffuse to the non-CHMO patients in the intervention practices. Results for non-CHMO patients (Table 3) indicated that there was a significant (P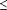 .05) effect for CBE and fecal occult blood slide examinations. In the intervention practices, the proportion of non-CHMO patients with a CBE in the previous 2 years increased from 26.6% at baseline to 36.5% at postintervention, while decreasing in the control practices. There was a positive net difference of 15.3%. For the fecal occult blood slide, the percentage of non-CHMO patients in the intervention practices with the test in the previous 2 years remained relatively similar at baseline and postintervention, 4.5% and 5.2%, respectively, while decreasing from 20.4% to 0.8% in the control group. This was a positive net difference of 20.3%. In both intervention and control practices, there were decreases in the proportions of non-CHMO patients with mammograms or Papanicolaou smears in the 2 years from baseline to postintervention. The decreases were smaller in the intervention practices, but the differences between the 2 groups were not significant. .05) effect for CBE and fecal occult blood slide examinations. In the intervention practices, the proportion of non-CHMO patients with a CBE in the previous 2 years increased from 26.6% at baseline to 36.5% at postintervention, while decreasing in the control practices. There was a positive net difference of 15.3%. For the fecal occult blood slide, the percentage of non-CHMO patients in the intervention practices with the test in the previous 2 years remained relatively similar at baseline and postintervention, 4.5% and 5.2%, respectively, while decreasing from 20.4% to 0.8% in the control group. This was a positive net difference of 20.3%. In both intervention and control practices, there were decreases in the proportions of non-CHMO patients with mammograms or Papanicolaou smears in the 2 years from baseline to postintervention. The decreases were smaller in the intervention practices, but the differences between the 2 groups were not significant.
Actual use of the chart reminder system by the practices was inferred from the presence of the intervention flow sheet in the medical charts in the postintervention chart abstractions. In the intervention practices, 12 practices (50%) had flow sheets in 75% or more of the CHMO charts, and 9 practices (37.5%) had flow sheets in 31% to 74% of the CHMO charts. The control practices used the flow sheet to a greater extent than expected: almost one fourth of the CHMO charts in the control practices had flow sheets. However, utilization of flow sheets in the control group was concentrated in only 8 practices. The remaining 15 practices had none or only a few flow sheets in the patient charts.
COMMENT
WAS THE INTERVENTION EFFECTIVE?
The intervention components used in this project were adapted from strategies found to be effective in previous studies. For example, McPhee et al19 found that office reminders and feedback to physicians increased the percentage of patients with a mammogram in the previous 1 year by about 17%, with a CBE by about 18%, and with a fecal occult blood slide by 15%. Similarly, Dietrich et al17 achieved increases of about 18% for mammography, 7% to 11% for CBE, and 4% to 13% for Papanicolaou smears with the use of chart reminders, feedback to physicians, and patient reinforcement. Tierney et al20 reported larger positive effects, up to 2- and 3-fold increases, in using cancer early-detection procedures, when computerized reminders were used at the time of the visit and the physicians were given periodic feedback.
Although direct comparisons of results across studies are difficult because of different intervention and evaluation methods, overall the results from our intervention are smaller and less consistent than those of previous studies. Our intervention resulted in significant positive outcomes for the Papanicolaou smear for CHMO patients, CBE for the non-CHMO patients, and the fecal occult blood slide for both patient groups. It did not have a positive effect on mammography.
The results of this intervention should be considered in light of the potential barriers existing in the study practices. The majority of the practices were small (54% had between 1 and 3 physicians) and most were located in socioeconomically disadvantaged neighborhoods. Many practices experienced high physician turnover and, during the 2-year study period, many had a decline in their total number of physicians.25 The difference between these settings and those where previous interventions were tested is best illustrated by comparing baseline screening rates. For example, Dietrich et al17 reported baseline rates of about 53% to 58% for a mammogram, 67% to 70% for CBE, and 58% to 63% for a Papanicolaou smear in the preceding 1 year. In contrast, we found baseline rates that were less than half of those for a 2-year period. Therefore, we believe that our intervention outcomes are encouraging because (1) they show that these intervention strategies can be implemented in community settings that present considerable obstacles to preventive care and (2) the intervention had significant positive effects on 3 of the 4 cancer detection procedures.
WAS THE INTERVENTION IMPLEMENTED SUCCESSFULLY?
The multiple components of the intervention (Table 1) were conceptualized to work as a unit through the existing CHMO-sponsored protocols. The degree to which the intervention was implemented successfully varied by components. Preparation and development of the CME seminar, on-site training and start-up assistance visits, mailing of the PHMC, QA visits, and feedback to the practices were ensured because either research and/or CHMO staff performed these components. The on-site training and start-up assistance were labor intensive. They required 3 personal visits to each intervention practice to ensure that all designated staff received training. The practices responded positively to these visits. Anticipating the second and third visits seemed to motivate staff to at least try to use the chart reminder system, and trying it generated questions that could be discussed at the subsequent visits. The intervention maintenance component was also easily incorporated into the ongoing QA system of CHMO and can continue indefinitely.
The CME and the PHMC did not work as intended. Few physicians attended the CME seminar; however, those who did attend rated it very favorably. Telephone interviews were conducted with a random sample of patients seen at the practices in the 3 months after the mailing of the cards. Results indicated that only 17% remembered receiving the card in the mail. However, patients who remembered receiving the card reported that they used the card to discuss cancer detection procedures with nurses or physicians at the practices. Strategies to increase physician participation in CME and to more efficiently disseminate patient devices such as the PHMC need to be explored further.
The chart reminder system was the principal tool that was intended to facilitate cancer screening as part of daily routines at the practices. Implementation of this component was dependent on personnel at the practices and was successful. Interest in the system was indicated by queries during site visits about using the form for non-CHMO patients, by requests for resupplies during the 2 years of the study, and by evidence in the medical charts of its continued use during the 2-year study period.
In summary, our experience indicates that most components of the tested intervention, especially its chart reminder system and QA with feedback to providers, are feasible for large-scale implementation, provided there is a medical intermediary who is willing to sponsor them.
OTHER RESULTS AND STUDY LIMITATIONS
Two additional topics—potential study limitations and the declining rates for mammography screening that we observed—warrant some discussion. The most significant factors that could have affected the results of this study are (1) incomplete and/or inaccurate medical records; (2) patients using other sources of health care; (3) comparability of the 4 study subgroups in terms of demographic characteristics, insurance coverage, and contact with the participating practices; and (4) contemporaneous events that we were not aware of. First, it is possible that incomplete records could have deflated CBE rates if physicians subsumed this test under other entries, such as "complete general checkup." However, it is unlikely that poor record keeping would have affected our estimates of screening rates for the other 3 examinations, since they would have resulted in separate laboratory reports in the medical charts. Second, it is unlikely that patients at the participating practices received cancer detection tests from other providers. Members of CHMO can only be referred to other providers by their primary care physicians. Moreover, the high mean number of visits and years at the practices suggest that these practices are the patients' regular sources of care. Third, data shown in Table 2 indicate that the 4 study subgroups were comparable on key measures of demographic characteristics, insurance status, and contact with the practices. The relatively small differences in the numbers of visits and years at the practices between the 2 intervention subgroups (baseline and postintervention) should not have affected the opportunity to receive cancer screening procedures. If they did, they would have reduced the chance of finding a significant intervention effect. Finally, we believe that events that we were unaware of may have affected screening rates for the fecal occult blood slide test. The large postintervention decline in the proportion of non-CHMO patients in the control practices with a fecal occult blood slide test is partly the result of the high rate observed at baseline. The 20.4% rate in this group was far out of range with all other observations for this test and suggests exposure to a 1-time colon cancer screening drive or some other unusual initiative.
The final topic for discussion concerns 2 findings from this study that are troubling and need further research. First, part of the positive effect of the intervention was caused by declining screening rates in the control practices, rather than increases in screening rates at the intervention sites. There was an overall decline in the proportion of patients screened between baseline and postintervention in the non-CHMO patient control group (see Table 3). The screening rates for this group declined for all 4 cancer detection procedures.
The second troubling finding is that the rates of mammography screening declined in all 4 study groups, and the largest net decline occurred in the intervention practices. We do not believe this is a result of the intervention. The intervention did not require the physicians to complete any extra forms or administrative procedures. Two events during the study period could account, at least in part, for these findings. One, the debate about the benefits of mammography for women younger than 50 years started during this study and has only been resolved recently. This debate might have negatively affected physicians' or patients' attitudes toward the examination. However, there is no reason why intervention practices should have been affected more than the control practices. A second possible explanation is the recent attention and debate focused on the current health care system. Issues of health care cost and insurance coverage, especially issues concerning public insurance programs, are at the forefront of these discussions. Physicians participating in this study may have been particularly sensitive to the prospects of major changes in public insurance, because almost half of the patients in their practices have public insurance. Perhaps the uncertainty about potential changes created more instability in their already hectic and stressed community practices. During the study period (1991-1994), a number of practices showed signs of instability: 10% of the initial sample of practices either closed or changed their patient clientele, and physician turnover across practices was high.25 Mammography differs from the 3 other detection procedures studied in that it is the most expensive and it requires referral to another provider. Instability in the larger health care system and in physicians' practices as well as uncertainty about public health insurance may have contributed to declining mammography screening rates that we observed. Again, however, these factors do not explain why the largest net decline occurred in the intervention practices.
CONCLUSIONS
A number of research studies conducted mostly in academic facilities or in a few other special settings have identified effective intervention strategies for promoting early cancer detection in physician offices. However, methods for disseminating these strategies to private community practices, especially those serving at-risk populations, have been lacking. This study evaluated an intervention designed to improve cancer screening in private practices serving low-income and minority populations that were not receiving regular cancer detection procedures. The baseline chart abstraction data confirmed the need for interventions to improve cancer screening in this population. The screening rates we observed were very low. They were considerably lower than those found in similar studies conducted in other physician settings or in those reported in the National Health Interview Survey among comparable populations.2-3
We found that dissemination of this intervention is feasible and reasonably well accepted in community physician practices. The intervention had a significant positive effect on Papanicolaou smears and fecal occult blood slide tests, and possibly on CBE, although results for the last were significant for only 1 of the 4 study subgroups. No intervention benefit was evident for mammography, and mammography screening rates declined in all 4 study subgroups. These discordant findings suggest a need for studying why mammography did not respond to the intervention to develop a more effective approach.
Finally, the intervention's multiple components and repeated, persistent, and personalized dissemination efforts were necessary to convey to the practices the importance of instituting the new program and to promote compliance with the protocols. This would not have been possible without strong and explicit CHMO sponsorship. The full commitment of an influential medical intermediary was indispensable for implementing the intervention and maintaining it over time. Overall, these findings demonstrate both the feasibility and the potential benefits of implementing this type of intervention through managed care organizations. The study showed that managed care organizations can give greater attention to, and provide incentives for, improving cancer screening of their members, regardless of the type of practice in which they are seen.
AUTHOR INFORMATION
Accepted for publication August 12, 1997.
This research was conducted under a grant from the National Cancer Institute, Bethesda, Md ("Prescribe for Health in Urban, Minority, Primary Care Physician Practices," NCI CA54403).
The study was made possible by the cooperation of Chicago HMO of United Health Care of Illinois Inc. We thank the physicians, nurses, and other personnel at the study practices for their patience and cooperation.
Reprints: Clara Manfredi, PhD, Health Research and Policy Centers, University of Illinois at Chicago, 850 W Jackson Blvd, Suite 400, Chicago, IL 60607-3025 (e-mail: clara.manfredi{at}uic.edu).
From the Health Research and Policy Centers (Drs Manfredi, Warnecke, and Lacey) and Department of Epidemiology and Biostatistics (Dr Freels), School of Public Health, University of Illinois at Chicago; the Department of Sociology and Anthropology, North Carolina State University, Raleigh (Dr Czaja); and Chicago HMO of United Health Care of Illinois Inc (Dr Trubitt).
Dr Lacey was the initial principal investigator of the research project that generated the data we report. She contributed substantially to earlier drafts of this article, which is based on the work she initiated and guided. Dr Lacey died on May 31, 1994.
REFERENCES
1. Miller B, Ries L, Hankey B, et al. SEER Cancer Statistics Review 1973-1990. Bethesda, Md: National Cancer Institute; 1993. NIH publication 93-2789.
2. Centers for Disease Control and Prevention. Update: National Breast and Cervical Cancer Early Detection Program, 1992-1993. MMWR Morb Mortal Wkly Rep. 1993;42:746-748.
3. Health Status of Minorities and Low-income Groups.3rd ed. Washington, DC; US Dept of Health and Human Services; 1991. Publication 271-848/40085.
4. Wells BL, Horm JW. Stage at diagnosis in breast cancer: race and socioeconomic factors. Am J Public Health. 1992;82:1383-1385.
WEB OF SCIENCE
| PUBMED
5. Belcher DW, Berg AO, Inui TS. Practical approaches to providing better preventive care: are physicians a problem or a solution? In: Battista R, Lawrence R, eds. Implementing Preventive Services. New York, NY: Oxford University Press; 1988:27-48.
6. American Cancer Society. Survey of physicians' attitudes and practices in early cancer detection. CA Cancer J Clin. 1990;40:77-101.
WEB OF SCIENCE
| PUBMED
7. Fink DJ. Cancer detection: the cancer-related checkup guidelines. In: Holleb AI, Fink DJ, Murphy GP, eds. American Cancer Society Textbook of Clinical Oncology. Atlanta, Ga: American Cancer Society Inc; 1991:153-176.
8. National Cancer Institute. Working Guidelines for Early Cancer Detection: Rationale and Supporting Evidence to Decrease Mortality. Bethesda, Md: Division of Cancer Prevention and Control, Early Detection Branch, National Cancer Institute; 1987.
9. McPhee S, Richard R, Solkowitz S. Performance of cancer screening in a university general internal medicine practice: comparison with 1980 American Cancer Society guidelines. J Gen Intern Med. 1986;1:275-281.
WEB OF SCIENCE
| PUBMED
10. Orlandi MA. Promoting health and preventing disease in health care settings: an analysis of barriers. Prev Med. 1987;16:119-130.
FULL TEXT
|
WEB OF SCIENCE
| PUBMED
11. Pommerenke FA. Implementing and maintaining preventive services: practical principles for primary care. J Fam Pract. 1992;34:92-97.
WEB OF SCIENCE
| PUBMED
12. NCI Breast Cancer Screening Consortium. Screening mammography: a missed clinical opportunity? JAMA. 1990;264:54-58.
FREE FULL TEXT
13. Resnicow K, Schorow M, Bloom H, Massad R. Obstacles to family practitioners' use of screening tests: determinants of practices. Prev Med. 1989;18:101-112.
FULL TEXT
|
WEB OF SCIENCE
| PUBMED
14. Warnecke RB. Intervention in black populations. In: Mettlin C, Murphy G, eds. Cancer Among Black Populations: Proceedings of the International Conference on Cancer Among Black Populations Held at Rosswell Park Memorial Institute, Buffalo, New York, May 5-6, 1980. New York, NY: Alan R Liss Inc; 1981:167-183.
15. Gemson D, Elinson J. Prevention in primary care: variability in physician practice patterns in New York City. Am J Prev Med. 1986;2:226-234.
WEB OF SCIENCE
| PUBMED
16. Escarce JJ, Epstein KR, Colby DC, Schwartz SJ. Racial differences in the elderly's use of medical procedures and diagnostic tests. Am J Public Health. 1993;83:948-954.
WEB OF SCIENCE
| PUBMED
17. Dietrich AJ, O'Connor GT, Keller A, Carney PA, Levy D, Whaley FS. Cancer: improving early detection and prevention: a community practice randomized trial. BMJ. 1992;304:687-691.
18. McDonald CJ, Hui SL, Smith DM, et al. Reminders to physicians from an introspective computer medical record. Ann Intern Med. 1984;100:130-138.
19. McPhee SJ, Bird JA, Jenkins C, Fordham D. Promoting cancer screening: a randomized, controlled trial of three interventions. Arch Intern Med. 1989;149:1866-1872.
FREE FULL TEXT
20. Tierney WM, Hui SL, McDonald CJ. Delayed feedback of physician performance versus immediate reminders to perform preventive care. Med Care. 1986;24:659-666.
FULL TEXT
|
WEB OF SCIENCE
| PUBMED
21. Lomas J, Haynes B. A taxonomy and critical review of tested strategies for the application of clinical practice recommendations: from "official" to "individual" clinical policy. In: Battista R, Lawrence R, eds. Implementing Preventive Services. New York, NY: Oxford University Press; 1988:77-94.
22. Carney PA, Dietrich AJ, Keller A, Landgraf J, O'Connor GT. Tools, teamwork, and tenacity: an office system for cancer prevention. J Fam Pract. 1992;35:388-394.
WEB OF SCIENCE
| PUBMED
23. Davidson R, Fletcher S, Retchin S, Duh S. A nurse-initiated reminder system for the periodic health examination: implementation and evaluation. Arch Intern Med. 1984;144:2167-2170.
FREE FULL TEXT
24. Sloane P, Rizzolo P, Citron D, et al. Implementation of recommended health maintenance activities in geriatric care. Fam Med. 1985;17:140-143.
PUBMED
25. Czaja R, Manfredi C, Warnecke R. Collecting survey and medical record data to measure intervention outcomes in medical practices serving urban minorities. In: Warnecke R, ed. Health Survey Research Methods. Hyattsville, Md: National Center for Health Statistics; 1996:209-214.
RELATED ARTICLES
Cancer Screening: A Challenge in Today's Changing Practice of Medicine
Roselyn Payne Epps
Arch Fam Med. 1998;7(4):315-316.
FULL TEXT
Can We Change Physicians' Practices in the Delivery of Cancer-Preventive Services?
Mack T. Ruffin IV
Arch Fam Med. 1998;7(4):317-319.
FULL TEXT
Cancer Early-Detection Services in Community Health Centers for the Underserved: A Randomized Controlled Trial
Allen J. Dietrich, Jonathan N. Tobin, Carol Hill Sox, Andrea N. Cassels, Fredeswinda Negron, Richard G. Younge, Neal A. Demby, and Tor D. Tosteson
Arch Fam Med. 1998;7(4):320-327.
ABSTRACT
| FULL TEXT
A Patient-Initiated System for Preventive Health Care: A Randomized Trial in Community-Based Primary Care Practices
Robert B. Williams, Myde Boles, and Robert E. Johnson
Arch Fam Med. 1998;7(4):338-345.
ABSTRACT
| FULL TEXT
Physician and Patient Predictors of Health Maintenance Visits
John S. Preisser, Stuart J. Cohen, James L. Wofford, William P. Moran, Brent J. Shelton, Maureen W. McClatchey, and Pamela Wolfe
Arch Fam Med. 1998;7(4):346-351.
ABSTRACT
| FULL TEXT
THIS ARTICLE HAS BEEN CITED BY OTHER ARTICLES
 |
Quebec breast cancer screening program: A study of the perceptions of physicians in Laval, Que
Nguyen et al.
cfp 2009;55:614-620.
ABSTRACT
| FULL TEXT
Influence of Primary Care Use on Population Delivery of Colorectal Cancer Screening
Fenton et al.
Cancer Epidemiol. Biomarkers Prev. 2009;18:640-645.
ABSTRACT
| FULL TEXT
Prompting Clinicians about Preventive Care Measures: A Systematic Review of Randomized Controlled Trials
Dexheimer et al.
J Am Med Inform Assoc 2008;15:311-320.
ABSTRACT
| FULL TEXT
Interventions to Enhance Breast Cancer Screening, Diagnosis, and Treatment among Racial and Ethnic Minority Women
Masi et al.
Med Care Res Rev 2007;64:195S-242S.
ABSTRACT
Long-term Results From a Randomized Controlled Trial to Increase Cancer Screening Among Attendees of Community Health Centers
Roetzheim et al.
Ann Fam Med 2005;3:109-114.
ABSTRACT
| FULL TEXT
Detection of Chronic Kidney Disease With Laboratory Reporting of Estimated Glomerular Filtration Rate and an Educational Program
Akbari et al.
Arch Intern Med 2004;164:1788-1792.
ABSTRACT
| FULL TEXT
A Randomized Controlled Trial to Increase Cancer Screening Among Attendees of Community Health Centers
Roetzheim et al.
Ann Fam Med 2004;2:294-300.
ABSTRACT
| FULL TEXT
Cancer Prevention Services and Physician Consensus in Primary Care Group Practices
Love et al.
Cancer Epidemiol. Biomarkers Prev. 2004;13:958-966.
ABSTRACT
| FULL TEXT
Effect of a Controlled Feedback Intervention on Laboratory Test Ordering by Community Physicians
Bunting and van Walraven
Clin. Chem. 2004;50:321-326.
ABSTRACT
| FULL TEXT
Variations in Diabetes Care and the Influence of Office Systems
Ellerbeck et al.
American Journal of Medical Quality 2004;19:12-18.
ABSTRACT
Effectiveness of Interventions to Increase Papanicolaou Smear Use
Yabroff et al.
J Am Board Fam Med 2003;16:188-203.
ABSTRACT
| FULL TEXT
Interventions That Increase Use of Adult Immunization and Cancer Screening Services: A Meta-Analysis
Stone et al.
ANN INTERN MED 2002;136:641-651.
ABSTRACT
| FULL TEXT
Evidence to action: a tailored multifaceted approach to changing family physician practice patterns and improving preventive care
Lemelin et al.
CMAJ 2001;164:757-763.
ABSTRACT
| FULL TEXT
Effectiveness of Interventions Designed to Increase Mammography Use: A Meta-Analysis of Provider-targeted Strategies
Mandelblatt and Yabroff
Cancer Epidemiol. Biomarkers Prev. 1999;8:759-767.
ABSTRACT
| FULL TEXT
Can we change physicians' practices in the delivery of cancer-preventive services?
Epps
Arch Fam Med 1998;7:315-316.
FULL TEXT
Can We Change Physicians' Practices in the Delivery of Cancer-Preventive Services?
Ruffin IV
Arch Fam Med 1998;7:317-319.
FULL TEXT
|