 |
 |

Failure of Treatment With Cephalexin for Lyme Disease
John Nowakowski, MD;
Donna McKenna, ANP;
Robert B. Nadelman, MD;
Denise Cooper, BS;
Susan Bittker, MS;
Diane Holmgren, RN;
Charles Pavia, PhD;
Russell C. Johnson, PhD;
Gary P. Wormser, MD
Arch Fam Med. 2000;9:563-567.
ABSTRACT
 |  |
Context Lyme disease typically presents with a skin lesion called erythema migrans (EM), which though often distinctive in appearance may be confused with cellulitis. The first-generation cephalosporin, cephalexin monohydrate, is effective for treating bacterial cellulitis but has not been recommended or studied for treating Lyme disease because of poor in vitro activity.
Objective To describe the outcome of patients with EM who were treated with cephalexin.
Patients and Methods Patients presenting with EM to the Lyme Disease Diagnostic Center in Westchester, NY (May 1992-September 1997). A 2-mm punch biopsy specimen of the leading edge of the EM lesion and/or blood was cultured for Borrelia burgdorferi.
Results Eleven (2.8%) of 393 study patients had been initially treated with cephalexin prior to our evaluation; 9 (82%) were originally diagnosed with cellulitis. Cephalexin was administered for 8.6 days (range, 2-21 days) prior to presentation. All 11 patients had clinical evidence of disease progression, including 8 whose rash enlarged, 2 who developed seventh-nerve palsy (1 with new EM lesions), and 1 who developed new EM lesions. Borrelia burgdorferi grew in cultures from 5 patients despite a mean of 9.8 days of treatment with cephalexin (range, 5-21 days).
Conclusion Cephalexin should not be used to treat early Lyme disease and should be prescribed with caution during the summer months for patients believed to have cellulitis in locations where Lyme disease is endemic.
INTRODUCTION
CEPHALEXIN is an oral first-generation cephalosporin antibiotic with good in vitro activity against Streptococcus pyogenes and many strains of Staphylococcus aureus.1 In addition, cephalexin is relatively inexpensive and well tolerated. For these reasons, it is often prescribed for the treatment of patients with bacterial cellulitis.2
Borrelia burgdorferi infection of the skin in early Lyme disease is associated with a skin lesion called erythema migrans (EM). When central clearing or a targetlike appearance is present, the lesion is unlikely to be confused with cellulitis caused by S pyogenes or S aureus. However, misdiagnosis is possible for patients with EM who do not present with classic features.3-4 Such patients may be inadvertently treated with cephalexin, an antimicrobial not recommended for this indication. We describe 11 such patients with EM who were initially treated with cephalexin.
PATIENTS, MATERIALS, AND METHODS
PATIENT ENROLLMENT AND B BURGDORFERI ANTIBODY TESTING
Patients seen at the walk-in Lyme Disease Diagnostic Center, Westchester Medical Center, Westchester, NY, between May 1, 1992, and September 30, 1997, with a rash consistent with EM were evaluated for entry into a diagnostic research protocol involving the cultivation of B burgdorferi from skin biopsy specimens or blood. This protocol was approved by the New York Medical College Institutional Review Board for research on human subjects. Those enrolled, after giving written informed consent, underwent a comprehensive history and physical examination. Blood was obtained at baseline and 10 to 20 days later for a polyvalent enzyme-linked immunosorbent assay (ELISA) to measure antibodies to B burgdorferi (Whittaker STAT ELISA kit; Whittaker Bioproducts Inc, Walkersville, Md). Samples were tested and interpreted according to the manufacturer's directions.
B BURGDORFERI CULTURE SPECIMENS
A 2-mm punch biopsy specimen was obtained from the leading edge of the EM lesion and placed into "incomplete" Barbour-Stoenner-Kelly (BSK) media.5 In certain patients, heparinized whole blood, serum, or plasma was also obtained for culture of B burgdorferi. Within 2 to 3 hours of collection, both skin- and blood-derived specimens were placed into BSK medium, incubated, and inspected for growth as previously described.6
IN VITRO SUSCEPTIBILITY
Minimum inhibitory concentrations (MIC) were determined using the microdilution method (96-well plate). Borrelia burgdorferi cultures in the log phase of growth were counted using a Petroff-Hausser (Hausser Scientific, Horsham, Pa) counting chamber. Duplicate wells containing BSK medium with and without the appropriately diluted antimicrobial agents were inoculated to a final density of 1x105 cells/mL of the test organism. After incubation at 34°C for 1 week, the wells were examined by dark-field microscopy for the presence of spirochetes, and the MIC was determined. All wells with negative findings (no spirochetes observed) were transferred (10% vol/vol) to BSK medium without antibiotics, incubated at 34°C for 3 weeks, and examined for spirochetes. The minimum bactericidal concentration (MBC) was the lowest antibiotic concentration from which spirochetes could not be subcultured.
RESULTS
PATIENT CHARACTERISTICS
Between May 1992, and September 1997, 393 patients with EM entered a Lyme disease research protocol. Eleven patients (2.8%) with EM were taking or had completed a course of cephalexin at the time of presentation. The clinical characteristics of these 11 patients are summarized in Table 1. In 9 (82%) of 11 patients, the prescribing clinician had diagnosed cellulitis. Eight of these 9 patients presented with atypical features of EM, which presumably led to this diagnosis. Six had tenderness at the rash site. Two had pustular or vesicular areas in the rash (Figure 1), and 1 had a uniformly erythematous lesion near the site of a previous mastectomy and lymph node dissection. Cephalexin was administered for an average of 8.6 days (range, 2-21 days) prior to presentation, with 5 patients completing the entire course of therapy prescribed (10-21 days) for the rash. Two of 11 patients had a history of penicillin allergy. Eight patients sought care at the Lyme Disease Diagnostic Center because their rash increased in size and/or systemic complaints such as arthralgias or fever developed while they were taking cephalexin. One patient developed a facial nerve palsy, and another developed a facial nerve palsy along with multiple new EM lesions, both within 10 days of completing a prescribed course of cephalexin for 21 and 10 days, respectively. Another patient developed multiple EM lesions after taking cephalexin for 9 days.
|
|

|
|
Table 1. Clinical and Laboratory Findings of 11 Patients With Erythema Migrans Who Were Initially Treated With Cephalexin*
|
|
|
|
|

|
|
Patient No. 4. Erythema migrans rash with atypical features. Note the uniform erythema with multiple vesicles surrounding the central punctum.
|
|
|
All patients were retreated with doxycycline hyclate with resolution of their rash and systemic symptoms. One patient had a slight residual facial palsy at 1 month and was then lost to follow-up. The average duration of follow-up for all 11 patients was 13.6 months (range, 1-30 months).
LABORATORY FEATURES
Nine (82%) of 11 patients had laboratory confirmation of B burgdorferi infection. Five (45%) of 11 had cultures positive for B burgdorferi, 3 from skin specimens alone, 1 from skin and blood specimens, and 1 from blood specimen alone. These 5 patients had taken cephalexin for an average of 9.8 days (range, 5-21 days) before the specimen was obtained that grew B burgdorferi. An additional patient whose culture was negative for bacteria had B burgdorferi detected by polymerase chain reaction from a skin biopsy specimen. Ten of 11 patients had baseline and follow-up ELISAs for antibodies to B burgdorferi performed. Four (40%) of 10 were positive at baseline and 3 seroconverted by their 10- to 20-day follow-up visit. One patient did not have an ELISA performed because of participation in a vaccine efficacy trial. Acute and convalescent immunoblots for B burgdorferi antibodies obtained as part of that trial were nondiagnostic.
MIC/MBC TESTING
Table 2 gives the results of MIC and MBC tests comparing cephalexin, cefuroxime axetil, ceftriaxone sodium, and amoxicillin with 7 strains of B burgdorferi. Two of the strains tested were the standard laboratory strains B31 (Ixodes scapularis tick isolate from New York) and B297 (human spinal fluid isolate). Five additional strains had been isolated from patients evaluated at the Lyme Disease Diagnostic Center, including 1 (B230) from patient No. 7. The MIC for cephalexin ranged from 25 µg/mL to 50 µg/mL while the MBC for cephalexin was more than 100 µg/mL in 6 of 7 strains tested.
|
|

|
|
Table 2. Minimum Inhibitory Concentration and Minimum Bactericidal Concentration Testing
|
|
|
COMMENT
Erythema migrans is the presenting manifestation in more than 85% of patients with objective evidence of Lyme disease.7 Typically, the erythema begins as a macule or papule at the site of the tick bite, which expands in size for days to weeks. Often described as a bull's-eye rash, central clearing is present in fewer than 40% of patients with EM.7 Atypical features may include central vesiculation and/or pustule formation, ecchymotic appearance, intense erythema with tenderness and warmth, or induration.8 Serologic testing in this early stage is often not helpful.7 Recommended therapy for this stage of Lyme disease includes oral doxycycline, amoxicillin, or cefuroxime for 14 days.7
Previous reports of in vitro susceptibility testing using a few strains of B burgdorferi9-10 and a single report of in vivo susceptibility in a mouse model using a Bor relia garinii strain, HP1,11 suggested that first-generation cephalosporins would be ineffective for treating Lyme disease in humans. Our study confirms and extends these findings. Based on in vitro study of 7 strains of B burgdorferi, including 5 recent clinical isolates, the MIC for the first-generation cephalosporin, cephalexin, was 25 µg/mL to 50 µg/mL, and the MBC was usually greater than 100 µg/mL. The MIC cutoff for susceptibility to cephalexin for other bacteria is less than or equal to 8 µg/mL; an MIC of 16 µg/mL is deemed moderately susceptible. The usual peak serum level of cephalexin after a 500-mg dose is 18 µg/mL.12 Therefore, cephalexin is inactive against B burgdorferi in vitro and would not be predicted to be clinically effective based on achievable blood levels.
These observations are in sharp contrast to certain second- or third-generation cephalosporins and to amoxicillin. For example, the MIC for cefuroxime, a second-generation cephalosporin, was less than or equal to 0.18 µg/mL; the MIC for the third-generation cephalosporin, ceftriaxone, was 0.02 µg/mL; and the MIC for amoxicillin was less than or equal to 0.25 µg/mL.10 Furthermore, all 3 of these agents are known to be clinically effective for patients with Lyme disease.
Susceptibility testing for B burgdorferi, however, is not standardized and has not always correlated with clinical efficacy. For example, macrolide antibiotics such as erythromycin base, azithromycin, and roxithromycin show effective inhibition and killing of B burgdorferi in vitro13-15 but have not been as successful as 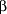 -lactam antibiotics or tetracyclines in animal models13, 16-17 or in clinical trials in humans.18-20 -lactam antibiotics or tetracyclines in animal models13, 16-17 or in clinical trials in humans.18-20
The findings of this study indicate that the measured in vitro activity of cephalexin and the clinical response to therapy are concordant. All 11 patients who were treated with cephalexin for EM responded poorly to treatment (Table 1). All 11 patients had objective evidence of clinical failure such as an increase in size of the rash (8 patients), development of new EM lesions (2 patients), and/or occurrence of a new seventh-nerve palsy (2 patients). In addition, B burgdorferi could be recovered in culture specimens from 5 patients despite a mean of 9.8 days of treatment with cephalexin (range, 5-21 days). Recovery of B burgdorferi during or following therapy with amoxicillin or a tetracycline derivative of greater than 24 hours' duration has never occurred in our experience.5, 21
Our findings do not prove that cephalexin is uniformly ineffective for Lyme disease because of the potential for referral bias of those patients failing to improve with treatment. Nevertheless, our observations strongly suggest that cephalexin should not be used for treating patients with Lyme disease and should be used with caution during the summer months for patients with cellulitis in locations where Lyme disease is endemic. The finding that nearly 1 in 35 patients with EM enrolled in Lyme disease studies at our center was initially prescribed cephalexin suggests that the possibility for diagnostic error is substantial. When bacterial cellulitis caused by S pyogenes or S aureus cannot be distinguished from EM, cefuroxime or amoxicillin-clavulanate potassium would be a preferred choice of therapy, as these drugs are effective for either type of infection.
AUTHOR INFORMATION
Accepted for publication February 9, 2000.
This study was supported in part by the Centers for Disease Control and Prevention, Atlanta, Ga (cooperative agreements U50/CCU 210286 [Dr Nadelman] and U50/CCU 215347 [Drs Nowakowski and Ms McKenna]), and by grants RO1-AR41508 (Drs Nadelman, Wormser, and Nowakowski) and RO1-AR34744 (Dr Johnson) from the National Institute of Arthritis and Musculoskeletal and Skin Diseases, Bethesda, Md.
The contents of this report are solely the responsibility of the authors and do not necessarily represent the official views of any of the previously mentioned agencies.
The authors would like to thank Lisa Coleman, BS, for assistance in performing antibiotic susceptibility testing.
Reprints: John Nowakowski, MD, Westchester Medical Center, Division of Infectious Diseases, Room 209SE, Macy Pavilion, Valhalla, NY 10595 (e-mail: John_Nowakowski{at}nymc.edu).
From the Division of Infectious Diseases (Drs Nowakowski, Nadelman, and Wormser and Ms McKenna, Ms Cooper, Ms Bittker, and Ms Holmgren), Department of Medicine, Westchester Medical Center, New York Medical College, Valhalla; the Department of Microbiology (Dr Johnson), University of Minnesota, Minneapolis; and New York College of Osteopathic Medicine Microbiology Laboratory (Dr Pavia), New York Institute of Technology, Old Westbury.
REFERENCES
 |  |
1. Braun P, Tillotson JR, Wilcox C, Finland M. Cephalexin and cephaloglycin activity in vitro and absorption and urinary excretion of single oral dose in young adults. Applied Microbiol. 1968;16:1684-1694.
2. Kumar A, Murray DL, Hanna CB, et al. Comparative study of cephalexin hydrochloride and cephalexin monohydrate in the treatment of skin and soft tissue infections. Antimicrob Agents Chemother. 1988;32:882-885.
FREE FULL TEXT
3. Nadelman RB, Wormser GP. Erythema migrans and early Lyme disease. Am J Med. 1995;98(suppl 4A):15S-24S.
4. Feder HM, Whitaker DL. Misdiagnosis of erythema migrans. Am J Med. 1995;99:412-419.
FULL TEXT
|
ISI
| PUBMED
5. Nadelman RB, Nowakowski J, Forseter G, et al. Failure to isolate Borrelia burgdorferi after antimicrobial therapy in culture-documented Lyme borreliosis associated with erythema migrans: report of a prospective study. Am J Med. 1993;94:583-588.
FULL TEXT
|
ISI
| PUBMED
6. Schwartz I, Wormser GP, Schwartz JJ, et al. Diagnosis of early Lyme disease by polymerase chain reaction amplification and culture of skin biopsies from erythema migrans lesions. J Clin Microbiol. 1992;30:3082-3088.
FREE FULL TEXT
7. Nadelman RB, Wormser GP. Lyme borreliosis. Lancet. 1998;352:557-565.
FULL TEXT
|
ISI
| PUBMED
8. Goldberg NS, Forseter G, Nadelman RB, et al. Vesicular erythema migrans. Arch Dermatol. 1992;128:1495-1498.
FREE FULL TEXT
9. Wretlind B, Johnson RC, Hansen K, Preac-Mursic V. Antibiotic susceptibility of Borrelia burgdorferi in vitro and in animal models. Scand J Infect Dis. 1991;(suppl 77):143-144.
10. Agger WA, Callister SM, Jobe DA. In vitro susceptibilities of Borrelia burgdorferi to five oral cephalosporins and ceftriaxone. Antimicrob Agents Chemother. 1992;36:1788-1790.
FREE FULL TEXT
11. Fugita H, Yamada K, Kurita T, et al. In vitro and in vivo antibiotics susceptibility of Lyme disease borrelia isolated from the ixodid tick in Japan. J Dermatol. 1995;22:935-38.
PUBMED
12. Griffith RS, Black HR. Cephalexin. Med Clin North Am. 1970;54:1229-1244.
ISI
| PUBMED
13. Preac-Mursic V, Wilske B, Schierz G, et al. Comparative antimicrobial activity of the new macrolides against Borrelia burgdorferi. Eur J Clin Microbiol Infect Dis. 1989;8:651-653.
FULL TEXT
|
ISI
| PUBMED
14. Dever LL, Jorgensen JH, Barbour AG. Comparative in vitro activities of clarithromycin, azithromycin, and erythromycin against Borrelia burgdorferi. Antimicrob Agents Chemother. 1993;37:1704-1706.
FREE FULL TEXT
15. Johnson RC, Kodner C, Russell M, Girard D. In vitro and in vivo susceptibility of Borrelia burgdorferi to azithromycin. J Antimicrob Chemother. 1990;25(suppl A):33-38.
16. Johnson RC, Kodner C, Russell M. In vitro and in vivo susceptibility of the Lyme disease spirochete, Borrelia burgdorferi, to four antimicrobial agents. Antimicrob Agents Chemother. 1987;31:164-167.
FREE FULL TEXT
17. Mursic VP, Wilske B, Schierz G, et al. In vitro and in vivo susceptibility of Borrelia burgdorferi. Eur J Clin Microbiol. 1987;6:424-426.
FULL TEXT
|
ISI
| PUBMED
18. Steere AC, Hutchinson GJ, Rahn DW, et al. Treatment of the early manifestations of Lyme disease. Ann Intern Med. 1983;99:22-26.
19. Hansen K, Hovmark A, Lebech AM, et al. Roxithromycin in Lyme borreliosis: discrepant results of an in vitro and in vivo animal susceptibility study and a clinical trial in patients with erythema migrans. Acta Derm Venereol. 1992;72:297-300.
ISI
| PUBMED
20. Luft BJ, Dattwyler RJ, Johnson RC, et al. Azithromycin compared with amoxicillin in the treatment of erythema migrans. Ann Intern Med. 1996;124:785-791.
FREE FULL TEXT
21. Berger BW, Johnson RC, Kodner C, Coleman L. Cultivation of Borrelia burgdorferi from erythema migrans lesions and peri-lesional skin. J Clin Microbiol. 1992;30:359-361.
FREE FULL TEXT
RELATED ARTICLE
The Archives of Family Medicine Continuing Medical Education Program
Arch Fam Med. 2000;9(6):551-552.
FULL TEXT
THIS ARTICLE HAS BEEN CITED BY OTHER ARTICLES
 |
Clinical practice. Early Lyme disease.
Wormser
NEJM 2006;354:2794-2801.
FULL TEXT
Diagnosis of Lyme Borreliosis
Aguero-Rosenfeld et al.
Clin. Microbiol. Rev. 2005;18:484-509.
ABSTRACT
| FULL TEXT
In Vitro Susceptibility Testing of Borrelia burgdorferi Sensu Lato Isolates Cultured from Patients with Erythema Migrans before and after Antimicrobial Chemotherapy
Hunfeld et al.
Antimicrob. Agents Chemother. 2005;49:1294-1301.
ABSTRACT
| FULL TEXT
Efficacy of Short-Course Ceftriaxone Therapy for Borrelia burgdorferi Infection in C3H Mice
Pavia et al.
Antimicrob. Agents Chemother. 2002;46:132-134.
ABSTRACT
| FULL TEXT
Lyme Disease
Steere
NEJM 2001;345:115-125.
FULL TEXT
Efficacy of an Evernimicin (SCH27899) In Vitro and in an Animal Model of Lyme Disease
Pavia et al.
Antimicrob. Agents Chemother. 2001;45:936-937.
ABSTRACT
| FULL TEXT
|