 |
 |

Does Influenza Vaccination Exacerbate Asthma?
Analysis of a Large Cohort of Children With Asthma
Piotr Kramarz, MD;
Frank DeStefano, MD;
Paul M. Gargiullo, PhD;
Robert L. Davis, MD, MPH;
Robert T. Chen, MD;
John P. Mullooly, PhD;
Steve B. Black, MD;
Henry R. Shinefield, MD;
Kari Bohlke, ScD;
Joel I. Ward, MD;
Michael S. Marcy, MD;
for the Vaccine Safety Datalink Team
Arch Fam Med. 2000;9:617-623.
ABSTRACT
 |  |
Context Although influenza vaccination is recommended for children with asthma, only a minority are vaccinated. One reason for low influenza vaccine coverage among children with asthma may be concern that influenza vaccination may induce an exacerbation of asthma.
Objective To evaluate the safety of influenza vaccination in children with asthma, we studied the incidence of hospitalizations and emergency department visits for asthma following influenza vaccination.
Design Retrospective cohort study–analysis of population-based computerized medical and vaccination records.
Setting Four large health maintenance organizations on the West Coast of the United States.
Subjects Children with asthma 1 through 6 years of age, identified by search of computerized databases of medical encounters and pharmacy prescriptions.
Main Outcome Measures Exacerbations of asthma.
Results In unadjusted analyses vaccination was associated with high rates of asthma exacerbations. However, after adjusting for asthma severity using a self-control method, the incidence rate ratios of asthma exacerbations after vaccination were 0.58 (95% confidence interval, 0.36-0.95), 0.74 (95% confidence interval, 0.47-1.17), and 0.98 (95% confidence interval, 0.76-1.27) during the 3 influenza seasons.
Conclusions After controlling for asthma severity, we found that influenza vaccination does not result in acute asthma exacerbations in children. Concern about possible exacerbation of asthma is not a valid reason to not vaccinate children with asthma against influenza.
INTRODUCTION
ASTHMA IS a large and growing health problem,1-4 especially in children.5-8 It is the most common cause of hospitalization in children,9 with rates increasing in recent decades.10 Asthma may be precipitated by viral infections of the upper respiratory tract, including influenza.11-16 Influenza infection can cause exacerbations of wheezing14 and life-threatening bronchial obstruction in children.17 Health authorities in many countries recommend annual influenza vaccination for patients with asthma.18-20 Despite these recommendations, only a few children with asthma receive an annual influenza vaccination.21-24
One reason for low influenza vaccine coverage among children with asthma may be concern that influenza vaccination may induce an exacerbation of asthma.23, 25-26 The evidence that influenza vaccine exacerbates asthma is conflicting. Observational27-31 and placebo-controlled studies32-34 conducted to date have been small and the results have been inconsistent.
To evaluate the safety of influenza vaccination in children with asthma, we studied the incidence of hospitalizations and emergency department (ED) visits for asthma following influenza vaccination at 4 large health maintenance organizations (HMOs).
METHODS
We conducted a retrospective cohort study using the Vaccine Safety Datalink.35 The Vaccine Safety Datalink is a computerized, linked database on immunizations, medical encounters, and demographic information on more than 1 million children enrolled in 4 large HMOs on the West Coast of the United States. We studied 3 influenza seasons: 1993-1994, 1994-1995, and 1995-1996. We defined the period from October 1 through April 30 as the influenza season. At the time of our analysis, data were available from 3 HMOs for the 1993-1994 and 1994-1995 influenza seasons and from all 4 HMOs for the 1995-1996 influenza season. We did not have access to pharmacy prescription data for the fourth HMO for 1993-1994 or 1994-1995.
The study was restricted to children 1 through 6 years of age because of the difficulty in differentiating between asthma and bronchiolitis in infants younger than 1 year.36 Eligible children had to meet our asthma case definition before May 1 of the year in which the influenza season began and had to have been continuously enrolled in the HMO from at least May 1 through October 1 of that year.
ASTHMA CASE DEFINITION
To identify children with asthma, we searched computerized outpatient clinic, hospital, ED, and pharmacy files. A case had to meet 1 of the following criteria since the beginning of HMO membership: (1) at least 1 International Classification of Diseases, Ninth Revision (ICD-9)37 code 493 and at least 1 prescription for any asthma medication; or (2) at least 1 prescription for a 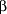 -agonist drug and at least 1 for cromolyn; or (3) 5 or more prescriptions for any asthma medication. This definition was adapted from a previous study conducted at one of the HMOs.38 Asthma medications included: -agonist drug and at least 1 for cromolyn; or (3) 5 or more prescriptions for any asthma medication. This definition was adapted from a previous study conducted at one of the HMOs.38 Asthma medications included: 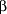 -agonists (inhaled or oral), theophyllin, corticosteroids (inhaled, oral, or injectable), cromolyn, adrenergic drugs not elsewhere specified, and unclassified asthma medications. -agonists (inhaled or oral), theophyllin, corticosteroids (inhaled, oral, or injectable), cromolyn, adrenergic drugs not elsewhere specified, and unclassified asthma medications.
STATISTICAL ANALYSIS
The main outcome measure was the number of acute asthma exacerbations, defined as hospitalizations or ED visits for asthma and identified from computerized HMO databases on medical encounters. Influenza vaccination data came from computerized vaccination databases. We computed incidence rates of asthma exacerbations by dividing the total number of exacerbations by the person-time contributed by each subject during periods of interest throughout each influenza season. In our primary analysis, we compared crude incidence rates of asthma exacerbations that occurred in vaccinated children within 2 weeks after vaccination to the background incidence rates of asthma exacerbations that occurred in unvaccinated children and in vaccinated children outside the postvaccination risk intervals. Since some children require 2 doses of vaccine to be fully immunized, we measured the 2-week postvaccination intervals after each dose of vaccine. We used a 2-week risk interval, but since some previous studies have reported asthma exacerbations within 48 hours after vaccination, we also evaluated a 2-day risk interval.
Controlling for severity of asthma in our analysis was very important to avoid "confounding by indication."39-41 This bias arises because the tendency to receive influenza vaccination increases with the severity of asthma.42 Such confounding, if uncontrolled, can result in a spurious association between influenza vaccination and acute asthma attacks. We used 2 different methods to control for confounding by indication. We first used unconditional Poisson regression models to estimate incidence rate ratios of asthma exacerbations adjusted for sex, age, HMO, severity of asthma, preventive care practices, and seasonal fluctuations in asthma exacerbations. We used the number of inhaled 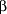 -agonist dispensings and the number of hospitalizations and ED visits for asthma during the 6 months before the influenza season (May 1 through September 30) to adjust for asthma severity. The use of -agonist dispensings and the number of hospitalizations and ED visits for asthma during the 6 months before the influenza season (May 1 through September 30) to adjust for asthma severity. The use of 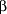 -agonists was categorized as 0, 1, 2, or 3 or more dispensings. Hospitalizations and ED visits were dichotomized (0 and -agonists was categorized as 0, 1, 2, or 3 or more dispensings. Hospitalizations and ED visits were dichotomized (0 and 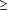 1). To adjust for preventive care practices, we used frequency of cromolyn dispensings (0 and 1). To adjust for preventive care practices, we used frequency of cromolyn dispensings (0 and 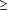 1) during the 6 months prior to the influenza season. 1) during the 6 months prior to the influenza season.
We conducted a second analysis using a self-control design, including only children who had at least 1 asthma exacerbation during the influenza season of interest. Each child was treated as a separate stratum, and incidence rates of asthma exacerbations within 2 weeks after vaccination were compared with other intervals for the same child. We used a conditional Poisson regression model43-44 to estimate incidence rate ratios. We included terms for 2-week intervals of calendar time throughout the entire influenza season to adjust for seasonal changes in asthma exacerbation incidence. Since the method uses individuals as their own controls, it implicitly controls for many potential confounders, such as asthma severity.43-45 We also performed self-control analysis using a 2-day risk interval. Separately, we performed a self-control analysis including only children with severe asthma (with 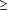 3 dispensings of a 3 dispensings of a 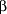 -agonist or at least 1 hospitalization or ED visit for asthma). -agonist or at least 1 hospitalization or ED visit for asthma).
We used the SAS GENMOD procedure for unconditional Poisson regression, and we wrote a special-purpose program using SAS Interactive Matrix Language for conditional Poisson regression.46
RESULTS
Table 1 gives, for each of the 3 influenza seasons, the number of children who met our asthma case definition and their demographic characteristics.
|
|

|
|
Table 1. Characteristics of Children With Asthma by Influenza Season, Vaccine Safety Datalink*
|
|
|
Among all children with asthma, the crude rates of asthma exacerbations (per 1000 child-months) during each influenza season were 5.00 (95% confidence interval [CI], 4.64-5.38) in 1993-1994, 4.61 (95% CI, 4.35-4.89) in 1994-1995, and 5.64 (95% CI, 5.43-5.86) in 1995-1996. In vaccinated children, the asthma attack rates showed no obvious relation to influenza vaccination (Figure 1).
|
|

|
|
Incidence rates of acute asthma attacks during weeks before and after influenza vaccination for 3 influenza seasons. The period from October 1 through April 30 was defined as the influenza season. Data were available from 3 health maintenance organizations (HMOs) for the 1993-1994 and 1994-1995 influenza seasons and from all 4 health maintenance organizations for the 1995-1996 influenza season. Access to pharmacy prescription data from the fourth health maintenance organization for 1993-1994 and 1994-1995 was unavailable.
|
|
|
In the first analysis, the crude incidence rates of asthma exacerbations within 2 weeks after vaccination were 2 to 3 times higher than the crude rates in unvaccinated children throughout the influenza season and in vaccinated children during periods outside the 2-week postvaccination interval (Table 2). After adjustment for sex, age, HMO, calendar time, asthma severity, and preventive care practices, the incidence rate ratios of asthma exacerbation within 2 weeks after influenza vaccination decreased but remained slightly elevated, and the increase in risk was statistically significant in the 1995-1996 season. Results using a 2-day risk interval were consistent with the 2-week interval results. The adjusted rate ratios of asthma exacerbation within 2 days after vaccination in the full cohort analysis were 0.55 (95% CI, 0.09-1.71), 1.34 (95% CI, 0.48-2.91), and 1.15 (95% CI, 0.60-1.98) in consecutive influenza seasons. The adjusted rate ratios of asthma exacerbation in children with severe asthma (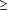 3 prescriptions for a 3 prescriptions for a 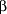 -agonist or with at least 1 hospitalization or ED visit) were 0.87 (95% CI, 0.39-1.66), 0.35 (95% CI, 0.11-0.83), and 1.34 (95% CI, 0.91-1.89) in consecutive influenza seasons. -agonist or with at least 1 hospitalization or ED visit) were 0.87 (95% CI, 0.39-1.66), 0.35 (95% CI, 0.11-0.83), and 1.34 (95% CI, 0.91-1.89) in consecutive influenza seasons.
|
|

|
|
Table 2. Full Cohort Analysis of Asthma Exacerbations Within 2 Weeks of Influenza Vaccination, by Influenza Season, Vaccine Safety Datalink
|
|
|
In the self-control analysis, in which the risk of asthma exacerbation in vaccinated children within 2 weeks after vaccination was compared with periods outside this interval, none of the incidence rate ratios were greater than 1.0 (Table 3). Using a 2-day risk interval, the adjusted rate ratios in the self-control analysis were 0.34 (95% CI, 0.08-1.37), 0.96 (95% CI, 0.40-2.36), and 0.83 (95% CI, 0.45-1.50) in each of the influenza seasons. The adjusted rate ratios of asthma exacerbation in children with severe asthma (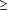 3 prescriptions for a 3 prescriptions for a 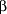 -agonist or with at least 1 hospitalization or ED visit) were 0.67 (95% CI, 0.41-1.14), 0.74 (95% CI, 0.44-1.23), and 1.02 (95% CI, 0.78-1.35) in consecutive influenza seasons. -agonist or with at least 1 hospitalization or ED visit) were 0.67 (95% CI, 0.41-1.14), 0.74 (95% CI, 0.44-1.23), and 1.02 (95% CI, 0.78-1.35) in consecutive influenza seasons.
|
|

|
|
Table 3. Self-control Analysis of Asthma Exacerbations During the 2-Week Period After Influenza Vaccination, by Influenza Season, Vaccine Safety Datalink
|
|
|
COMMENT
After controlling for confounding by asthma severity, we found that influenza vaccination did not lead to an acute exacerbation of asthma within 2 days or 2 weeks after vaccination. In the past, a few anecdotal reports and physiologic studies have sustained concerns about asthma exacerbation.23, 26 Several small studies failed to find any harmful effects on lung function after vaccination.29, 31, 47-49 However, 2 investigators have reported exacerbations of asthma following influenza vaccination.50-51 Furthermore, some authors described increased bronchial responsiveness after influenza vaccination.28, 52 Bell et al27 observed a significantly increased use of bronchodilating drugs and a decrease in the peak expiratory flow rate 48 hours after vaccination.
Results from controlled clinical trials also have been inconsistent. Campbell and Edwards53 in a crossover trial observed significant reductions in peak expiratory flow with no increase in symptoms during the week after influenza vaccination, but they examined adults and their sample size was small (28 individuals). Kava et al32 showed no increased need for bronchodilators up to 21 days after influenza immunization of 16 adults. Stenius-Aarniala et al33 in a study of 318 adults (161 with asthma) did not find a significant difference in clinical symptoms, medication use, or peak expiratory flow between vaccinated patients and placebo recipients. De Jongste et al30 showed increased bronchial responsiveness to histamine after administration of live, but not after inactivated, influenza vaccine to asthmatic children. Recently, Nicholson et al34 in a crossover study among 255 adults with asthma demonstrated a fall in peak expiratory flow after influenza vaccination, but the effect became insignificant after patients with common colds were removed from the analysis.
Regardless of possible subclinical physiological effects on pulmonary function, the results of our study indicate that influenza vaccination does not result in clinically meaningful exacerbations of asthma symptoms in children. The crude risk of asthma exacerbation within 2 weeks after influenza vaccination was 2 to 3 times higher than the risk in unvaccinated children or during periods outside the 2-week interval after vaccination. These crude results, however, were confounded by severity of asthma since children with more severe asthma are more likely to be vaccinated against influenza.42 After adjustment for asthma severity and other potential confounders in unconditional Poisson regression models, the incidence rate ratios of asthma exacerbation within 2 weeks after immunization decreased to between 1 and 1.39, depending on the influenza season. The relative risks for the 1993-1994 and 1994-1995 influenza seasons were not statistically significant; however, the relative risk for the 1995-1996 influenza season was significant (1.39; 95% CI, 1.08-1.77). Using a self-control method, which more fully adjusts for asthma severity, resulted in incidence rate ratios of asthma exacerbation that were all at or below 1.0. Almost all previous studies of the safety of influenza vaccination have had observation periods of 2 weeks or less after vaccination.27-28,30, 33-34 We also analyzed asthma exacerbations occurring within a 2-day risk interval, and we did not detect any increased risk of asthma exacerbation within this shorter interval. We also performed separate analyses of children with severe asthma and did not find an increased risk of asthma exacerbations following influenza vaccination.
The difference between the relative risks computed based on the traditional cohort analysis and the self-control analysis results from the differences in method. The 2 approaches analyze different populations. In the traditional cohort analysis, vaccinated children were compared with unvaccinated children. In the self-control analysis, only children with at least 1 asthma exacerbation were analyzed. In this method, the incidence of asthma exacerbations within the 2-week period after vaccination was compared with the incidence outside this risk interval in the same child. The self-control method adjusts for asthma severity on an individual level, using individuals as their own controls. The method controls for many other potential confounders that may be difficult to measure or that may not have been measured.43-44
Although ours was an observational study, it had many advantages over previous studies, including: (1) it was population based, (2) the sample size was large enough to detect even small differences in risk (eg, we could have detected a relative risk of 1.25 with 90% power), (3) the results were consistent across 3 different influenza seasons, and (4) there was rigorous statistical adjustment for asthma severity and other potential confounding factors.
There were, however, some limitations to our study. First, we know that there were some errors in our computerized vaccination data, but these were not large. Quality control analyses in the Vaccine Safety Datalink have shown that between 78% and 89% of influenza vaccinations recorded in the medical records were captured by the automated vaccination databases.54 Another concern might be that we used hospitalizations and ED visits as surrogate measures of asthma exacerbation, thus limiting our analysis to more severe exacerbations. Our asthma case definition could also be questioned. A universal asthma case definition is difficult to design,55 and others also have relied on ICD-9 codes36-37,56-58 and asthma medication data38, 56, 59 to define asthma cases. A case definition similar to ours was previously shown to have high sensitivity and positive predictive value.38
Another potential source of bias may have occurred if the vaccination was postponed in cases where a child had been recently hospitalized for asthma. To explore this possibility we computed the incidence of acute asthma attacks during 2-month periods before and after an influenza vaccination. The rates showed no obvious relation to influenza vaccination (Figure 1).
We used prescriptions for asthma medications as one of the measures of asthma severity. We believe that the number of prescriptions provides an accurate reflection of asthma severity because of the uniformity of asthma management in the HMOs. All the HMOs participating in the study have guidelines for asthma management that are reinforced through continual quality control monitoring.
We excluded children younger than 1 year in our analysis because of the difficulty in differentiating asthma and bronchiolitis in this age category. We have performed a subanalysis including these children, and the results were consistent with the results of the analysis of children 1 through 6 years of age (data not shown).
CONCLUSIONS
In this large, population-based, observational study, we found that influenza vaccination does not result in acute asthma exacerbations in children. Concern about possible exacerbation of asthma is not a valid reason to not vaccinate children with asthma against influenza. We observed a decrease in the incidence of asthma exacerbations within 2 weeks after influenza vaccination during all analyzed influenza seasons that was statistically significant during the 1993-1994 influenza season (relative risk 0.58; 95% CI, 0.36-0.95). The results raise the question whether influenza vaccination may cause a decrease in the incidence of asthma attacks beyond the 2 weeks (ie, vaccination may provide long-term protection against asthma exacerbations). We are exploring this possibility in a separate analysis.
AUTHOR INFORMATION
Accepted for publication February 2, 2000.
The authors have no commercial, proprietary, or financial interest in the products or companies described in this article.
Members of the Vaccine Safety Datalink Team
National Immunization Program, Centers for Disease Control and Prevention, Atlanta, Ga: Frank DeStefano, MD, MPH, John Glasser, PhD, MPH, Robert T. Chen, MD, MA, Piotr Kramarz, MD, Henry Rolka, RN, MPS, MS, David Walker, MPH, Catherine Okoro, MS, Paul M. Gargiullo, PhD, Drew Baughman, MS, Ruojiao Cao, David King. Group Health Cooperative of Puget Sound, Seattle, Wash: Robert S. Thompson, MD, Robert L. Davis, MD, MPH, Lisa Jackson, MD, MPH, Patti Benson, MPH, Virginia Immanuel, MPH, William Barlow, PhD, Kari Bohlke, ScD, Paula Lee Poy, Viviana Rebolledo, David Rubanowice, Loren Mell, Michelle LaFrance, Linda Huggins, RN, Jas Dhillon, Ann Zavitkovsky, MPH. Northwest Kaiser Permanente, Portland, Ore: John P. Mullooly, PhD, Lois Drew, Kim Olson, Jill Mesa, John A. Pearson, MD, Mike Allison, Alan Bauck, Nadia Redmond, MSPH. Northern California Kaiser Permanente, Oakland: Steven B. Black, MD, Henry R. Shinefield, MD, Paula Ray, MPH, Edwin Lewis, MPH, Bruce H. Fireman, MA, Tracy A. Lieu, MD. Center for Vaccine Research Harbor-UCLA Medical Center, Torrance: Joel I. Ward, MD, Constance M. Vadheim, PhD, Hang Lee, PhD, Jennie Jing, MA. Southern California Kaiser Permanente, Los Angeles: Nancy Goff, S. Michael Marcy, MD, Marlene Lugg, DrPH.
Corresponding author: Piotr Kramarz, MD, National Immunization Program, Centers for Disease Control and Prevention, 1600 Clifton Rd, MS-E61, Atlanta, GA 30333 (e-mail: pdk3{at}cdc.gov).
From the Centers for Disease Control and Prevention, Atlanta, Ga (Drs Kramarz, DeStefano, Gargiullo, and Chen); Center for Health Studies, Group Health Cooperative of Puget Sound, Seattle, Wash (Drs Davis and Bohlke); Pediatric Vaccine Study Center, Northern California Kaiser Permanente, Oakland (Drs Black and Shinefield); Center for Health Research, Northwest Kaiser Permanente, Portland, Ore (Dr Mullooly); Center for Vaccine Research Harbor-UCLA Medical Center, Torrance (Dr Ward); Kaiser Foundation Hospital, Panorama City, Calif (Dr Marcy).
REFERENCES
 |  |
1. Burr ML. Is asthma increasing? J Epidemiol Community Health. 1987;41:185-189.
FREE FULL TEXT
2. Balfe D, Crane J, Beasley R, Pearce N. The worldwide increase in the prevalence of asthma in children and young adults. Continuing Med Educ J. 1996;14:433-442.
3. Woolcock AJ, Peat JK. Evidence for the increase of asthma worldwide. In: The Rising Trends in Asthma: Ciba Foundation Symposium 206. New York, NY: John Wiley & Sons Inc; 1997:122-139.
4. Robertson CF, Dalton MF, Peat JK, et al. Asthma and other atopic diseases in Australian children: Australian arm of the International Study of Asthma and Allergy in Childhood. Med J Aust. 1998;168:434-438.
ISI
| PUBMED
5. Ninan TK, Russell G. Respiratory symptoms and atopy in Aberdeen schoolchildren: evidence from two surveys 25 years apart. BMJ. 1992;304:873-875.
6. Burr ML, Butland BK, King S, Vaughan-Williams E. Changes in asthma prevalence: two surveys 15 years apart. Arch Dis Child. 1989;64:1452-1456.
FREE FULL TEXT
7. Weitzman M, Gortmaker SL, Sobol AM, et al. Recent trends in the prevalence and severity of childhood asthma. JAMA. 1992;268:2673-2677.
FREE FULL TEXT
8. Anderson HR, Butland BK, Strachan DP. Trends in prevalence and severity of childhood asthma. BMJ. 1994;308:1600-1604.
FREE FULL TEXT
9. Halfon N, Newacheck PW. Trends in the hospitalization for acute childhood asthma, 1970-84. Am J Public Health. 1986;76:1308-1311.
FREE FULL TEXT
10. Evans III R, Mullally DI, Wilson RW, et al. National trends in the morbidity and mortality of asthma in the US: prevalence, hospitalization and death from asthma over two decades: 1965-1984. Chest. 1987;91(6 suppl):65S-74S.
11. Beasley R, Coleman ED, Hermon Y, et al. Viral respiratory tract infection and exacerbations of asthma in adult patients. Thorax. 1988;43:679-683.
FREE FULL TEXT
12. McIntosh K, Ellis EF, Hoffman LS, Lybass TG, Eller JJ, Fulginiti VA. Association of viral and bacterial respiratory infection with exacerbations of wheezing in young asthmatic children. Chest. 1973;63(suppl):43S.
13. Minor TE, Baker JW, Dick EC, et al. Greater frequency of viral respiratory infections in asthmatic children as compared with their nonasthmatic siblings. J Pediatr. 1974;85:472-477.
FULL TEXT
|
ISI
| PUBMED
14. Minor TE, Dick EC, Baker JW, Ouellette JJ, Cohen M, Reed CE. Rhinovirus and influenza type A infections as precipitants of asthma. Am Rev Respir Dis. 1976;113:149-153.
ISI
| PUBMED
15. Rothbarth PH, Kempen BM, Sprenger MJ. Sense and nonsense of influenza vaccination in asthma and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1995;151:1682-1685.
ABSTRACT
16. Johnston SL, Pattemore PK, Sanderson G, et al. The relationship between upper respiratory infections and hospital admissions for asthma: a time-trend analysis. Am J Respir Crit Care Med. 1996;154:654-660.
ABSTRACT
17. Kondo S, Abe K. The effects of influenza virus infection on FEV1 in asthmatic children. Chest. 1991;100:1235-1238.
FREE FULL TEXT
18. Centers for Disease Control and Prevention. Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 1997;46:1-25.
PUBMED
19. Expert Panel Report II: Guidelines for the Diagnosis and Management of Asthma. Bethesda, Md: National Asthma Education and Prevention Program; 1997. National Institutes of Health publication 97-4051.
20. American Academy of Pediatrics. Influenza. In: Peter G, ed. 1977 Red Book: Report of the Committee on Infectious Diseases. 24th ed. Elk Grove Village, Ill: American Academy of Pediatrics; 1997:307-315.
21. Szilagyi PG, Rodewald LE. Missed opportunities for vaccination among children with asthma. Pediatr Infect Dis J. 1992;11:705-708.
ISI
| PUBMED
22. Szilagyi PG, Rodewald LE, Savageau J, Yoos L, Doane C. Improving influenza vaccination rates in children with asthma: a test of a computerized reminder system and an analysis of factors predicting vaccination compliance. Pediatrics. 1992;90:871-875.
FREE FULL TEXT
23. Watson JM, Cordier JF, Nicholson KG. Does influenza immunization cause exacerbations of chronic airflow obstruction or asthma? Thorax. 1997;52:190-194.
ISI
| PUBMED
24. Department of Health Welsh Office Scottish Office Home and Health Department DHSS (Northern Ireland). Immunization Against Infectious Disease. London, England: Her Majesty's Stationery Office; 1992.
25. Park CL, Frank AL, Sullivan M, Jindal P, Baxter BD. Influenza vaccination of children during acute asthma exacerbation and concurrent prednisone therapy. Pediatrics. 1996;98(2, pt 1):196-200.
26. Park CL, Frank A. Does influenza vaccination exacerbate asthma? Drug Saf. 1998;19:83-88.
FULL TEXT
|
ISI
| PUBMED
27. Bell TD, Chai H, Berlow B, Daniels G. Immunization with killed influenza virus in children with chronic asthma. Chest. 1978;73:140-145.
FREE FULL TEXT
28. Banks J, Bevan C, Fennerty A, Ebden P, Walters EH, Smith AP. Association between rise in antibodies and increase in airway sensitivity after intramuscular injection of killed influenza virus in asthmatic patients. Eur J Respir Dis. 1985;66:268-272.
ISI
| PUBMED
29. Ghirga G, Ghirga P, Rodino P, Presti A. Safety of the subunit influenza vaccine in asthmatic children. Vaccine. 1991;9:913-914.
30. De Jongste JC, Degenhart HJ, Neijens HJ, Duiverman EJ, Raatgeep HC, Kerrebijn KF. Bronchial responsiveness and leucocyte reactivity after influenza vaccine in asthmatic patients. Eur J Respir Dis. 1984;65:196-200.
ISI
| PUBMED
31. Albazzaz MK, Harvey JE, Grilli EA, Caul EO, Roome AP. Subunit influenza vaccination in adults with asthma: effect on clinical state, airway reactivity, and antibody response. Br Med J (Clin Res Ed). 1987;294:1196-1197.
32. Kava T, Lindqvist A, Karjalainen J, Laitinen LA. Unchanged bronchial reactivity after killed influenza virus vaccine in adult asthmatics. Respiration. 1987;51:98-104.
ISI
| PUBMED
33. Stenius-Aarniala B, Huttunen JK, Pyhala R, et al. Lack of clinical exacerbations in adults with chronic asthma after immunization with killed influenza virus. Chest. 1986;89:786-789.
FREE FULL TEXT
34. Nicholson KG, Nguyen-Van-Tam JS, Ahmed AH, et al. Randomised placebo-controlled crossover trial on effect of inactivated influenza vaccine on pulmonary function in asthma. Lancet. 1998;351:326-331.
FULL TEXT
|
ISI
| PUBMED
35. Chen RT, Glasser JW, Rhodes PH, et al. Vaccine Safety Datalink project: a new tool for improving vaccine safety monitoring in the United States: the Vaccine Safety Datalink Team. Pediatrics. 1997;99:765-773.
FREE FULL TEXT
36. Lozano P, Fishman P, VonKorff M, Hecht J. Health care utilization and cost among children with asthma who were enrolled in a health maintenance organization. Pediatrics. 1997;99:757-764.
FREE FULL TEXT
37. World Health Organization. International Classification of Diseases, Ninth Revision (ICD-9). Geneva, Switzerland: World Health Organization; 1977.
38. Osborne ML, Vollmer WM, Johnson RE, Buist AS. Use of an automated prescription database to identify individuals with asthma. J Clin Epidemiol. 1995;48:1393-1397.
FULL TEXT
|
ISI
| PUBMED
39. Suissa S. The case-time-control design: further assumptions and conditions. Epidemiology. 1998;9:441-445.
FULL TEXT
|
ISI
| PUBMED
40. Grobbee DE, Hoes AW. Confounding and indication for treatment in evaluation of drug treatment for hypertension. BMJ. 1997;315:1151-1154.
FREE FULL TEXT
41. Edwardes MD, Baltzan M. Confounding by indication, histamine2-receptor antagonists, and gastric cancer. Epidemiology. 1997;8:335.
ISI
| PUBMED
42. Kramarz P, DeStefano F, Gargiullo PM, et al and the Vaccine Safety Datalink Team. Influenza vaccination in children with asthma in Health Maintenance Organizations. Vaccine. 2000;18:2288-2294.
FULL TEXT
|
ISI
| PUBMED
43. Farrington CP. Relative incidence estimation from case series for vaccine safety evaluation. Biometrics. 1995;51:228-235.
FULL TEXT
|
ISI
| PUBMED
44. Farrington CP, Nash J, Miller E. Case series analysis of adverse reactions to vaccines: a comparative evaluation. Am J Epidemiol. 1996;143:1165-1173. [published erratum appears in Am J Epidemiol. 1998;147:93].
FREE FULL TEXT
45. Not Available In comment of: Gargiullo PM, Kramarz P, DeStefano F, Chen RT. Principles of epidemiological research on drug effects. Lancet. 1999;353:501. Comment on: Lancet. 1998;352:1767-1770.
PUBMED
46. Changes and Enhancements Through Release 6.12. SAS/STAT Software. Cary, NC: SAS Institute Inc; 1997:1167.
47. Kava T. Acute respiratory infection, influenza vaccination and airway reactivity in asthma. Eur J Respir Dis Suppl. 1987;150:1-38.
PUBMED
48. Kava T, Laitinen LA. Effects of killed and live attenuated influenza vaccine on symptoms and specific airway conductance in asthmatics and healthy subjects. Allergy. 1985;40:42-47.
ISI
| PUBMED
49. Ahmed AH, Nicholson KG, Hammersley VS, Kent J. Influenza vaccination in patients with asthma: effect on peak expiratory flow, asthma symptoms and use of medication. Vaccine. 1997;15:1008-1009.
FULL TEXT
|
ISI
| PUBMED
50. Hassan WU, Henderson AF, Keaney NP. Influenza vaccination in asthma [letter]. Lancet. 1992;339:194.
51. Daggett P. Influenza and asthma [letter]. Lancet. 1992;339:367.
52. Ouellette JJ, Reed CE. Increased response of asthmatic subjects to methacholine after influenza vaccine. J Allergy. 1965;36:558-563.
FULL TEXT
|
ISI
| PUBMED
53. Campbell BG, Edwards RL. Safety of influenza vaccination in adults with asthma. Med J Aust. 1984;140:773-775.
ISI
| PUBMED
54. Mullooly J, Drew L, DeStefano F, et al. Quality of HMO vaccination databases used to monitor childhood vaccine safety: Vaccine Safety DataLink Team. Am J Epidemiol. 1999;149:186-194.
FREE FULL TEXT
55. Sears MR. Descriptive epidemiology of asthma. Lancet. 1997;350(suppl 2):SII1-SII4.
56. Lieu TA, Quesenberry CP Jr, Capra AM, Sorel ME, Martin KE, Mendoza GR. Outpatient management practices associated with reduced risk of pediatric asthma hospitalization and emergency department visits. Pediatrics. 1997;100:334-341.
FREE FULL TEXT
57. Griffiths C, Sturdy P, Naish J, Omar R, Dolan S, Feder G. Hospital admissions for asthma in east London: associations with characteristics of local general practices, prescribing, and population. BMJ. 1997;314:482-486.
FREE FULL TEXT
58. Campbell MJ, Cogman GR, Holgate ST, Johnston SL. Age-specific trends in asthma mortality in England and Wales, 1983-95: results of an observational study. BMJ. 1997;314:1439-1441.
FREE FULL TEXT
59. Spitzer WO, Suissa S, Ernst P, et al. The use of beta-agonists and the risk of death and near death from asthma. N Engl J Med. 1992;326:501-506.
ABSTRACT
RELATED ARTICLE
The Archives of Family Medicine Continuing Medical Education Program
Arch Fam Med. 2000;9(7):639-641.
FULL TEXT
THIS ARTICLE HAS BEEN CITED BY OTHER ARTICLES
 |
The methodology of self-controlled case series studies
Whitaker et al.
Stat Methods Med Res 2009;18:7-26.
ABSTRACT
Influenza Virus and Acute Asthma in Children: In Reply
Poehling et al.
Pediatrics 2008;121:1080-1080.
FULL TEXT
Influenza Burden for Children With Asthma
Miller et al.
Pediatrics 2008;121:1-8.
ABSTRACT
| FULL TEXT
The use of primary care databases: case-control and case-only designs
Smeeth et al.
Fam Pract 2006;23:597-604.
ABSTRACT
| FULL TEXT
Adverse Events After Inactivated Influenza Vaccination Among Children Less Than 2 Years of Age: Analysis of Reports From the Vaccine Adverse Event Reporting System, 1990-2003
McMahon et al.
Pediatrics 2005;115:453-460.
ABSTRACT
| FULL TEXT
Safety of the Trivalent Inactivated Influenza Vaccine Among Children: A Population-Based Study
France et al.
Arch Pediatr Adolesc Med 2004;158:1031-1036.
ABSTRACT
| FULL TEXT
AUTHOR'S REPLY
Goldstein
The Annals of Pharmacotherapy 2004;38:1324-1324.
FULL TEXT
Comment: safety and efficacy of influenza vaccine in children
van der Wouden and Bueving
The Annals of Pharmacotherapy 2004;38:1323-1324.
FULL TEXT
Population-Based Surveillance for Hospitalizations Associated With Respiratory Syncytial Virus, Influenza Virus, and Parainfluenza Viruses Among Young Children
Iwane et al.
Pediatrics 2004;113:1758-1764.
ABSTRACT
| FULL TEXT
Does influenza vaccination increase consultations, corticosteroid prescriptions, or exacerbations in subjects with asthma or chronic obstructive pulmonary disease?
Tata et al.
Thorax 2003;58:835-839.
ABSTRACT
| FULL TEXT
Potential Burden of Universal Influenza Vaccination of Young Children on Visits to Primary Care Practices
Szilagyi et al.
Pediatrics 2003;112:821-828.
ABSTRACT
| FULL TEXT
Asthma and Influenza Vaccination
Eisner
Chest 2003;124:775-777.
FULL TEXT
Matched Cohort Methods for Injury Research
Cummings et al.
Epidemiol Rev 2003;25:43-50.
FULL TEXT
Exposure to Tricyclic and Selective Serotonin Reuptake Inhibitor Antidepressants and the Risk of Hip Fracture
Hubbard et al.
Am J Epidemiol 2003;158:77-84.
ABSTRACT
| FULL TEXT
Addressing Parents' Concerns: Do Vaccines Cause Allergic or Autoimmune Diseases?
Offit and Hackett
Pediatrics 2003;111:653-659.
ABSTRACT
| FULL TEXT
Time Spent by Primary Care Practices on Pediatric Influenza Vaccination Visits: Implications for Universal Influenza Vaccination
Szilagyi et al.
Arch Pediatr Adolesc Med 2003;157:191-195.
ABSTRACT
| FULL TEXT
The Safety of Inactivated Influenza Vaccine in Adults and Children with Asthma
The American Lung Association Asthma Clinical Rese
NEJM 2001;345:1529-1536.
ABSTRACT
| FULL TEXT
Kemp and Wyckoff
AAP News 2000;17:134-135.
FULL TEXT
|