 |
 |

Physician and Patient Predictors of Health Maintenance Visits
John S. Preisser, PhD;
Stuart J. Cohen, EdD;
James L. Wofford, MD;
William P. Moran, MD;
Brent J. Shelton, PhD;
Maureen W. McClatchey, PhD;
Pamela Wolfe, MS
Arch Fam Med. 1998;7:346-351.
ABSTRACT
Background Because of a strong association between health maintenance visits (HMVs) and cancer screening, knowledge of the predictors of an HMV have implications for screening.
Objective To examine the association of an HMV with patient, physician, and practice characteristics in the primary care setting.
Design A statewide study of cancer screening was conducted in Colorado to determine concordance with the National Cancer Institute's guidelines for screening for breast, cervical, prostate, and skin cancer. Medical records from patients were randomly chosen from primary care practices. Predictors of an HMV were determined by fitting a logistic model to baseline data, adjusting for the cluster sampling of patients within practices.
Setting Nonacademic primary care practices in Colorado.
Participants A total of 5746 patients aged 42 to 74 years from 132 primary care practices.
Main Outcome Measure Whether a patient had an HMV in the previous year.
Results Of all patients, 31% had an HMV in the previous year. Patient characteristics associated with having HMVs included nonsmoking status, odds ratio (OR) (95% confidence interval [CI]) of 1.27 (1.11-1.46), age, and sex. Women aged 50 to 69 years were significantly more likely to have an HMV than men aged 50 to 69 years (OR, 1.30; 95% CI, 1.10-1.54). Among adults aged 70 years and older, there were no significant sex differences in receiving HMVs. Physician and practice characteristics associated with providing HMVs included practice size (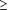 3 full-time physicians) (OR, 1.34; 95% CI, 1.01-1.77), physician contemplation of changing approaches to cancer screening (OR, 1.33; 95% CI, 1.04-1.70), and physician female sex (OR, 1.33; 95% CI, 1.04-1.70). Physician age and specialty (general internist or family physician) were not associated with the level of health maintenance delivery. 3 full-time physicians) (OR, 1.34; 95% CI, 1.01-1.77), physician contemplation of changing approaches to cancer screening (OR, 1.33; 95% CI, 1.04-1.70), and physician female sex (OR, 1.33; 95% CI, 1.04-1.70). Physician age and specialty (general internist or family physician) were not associated with the level of health maintenance delivery.
Conclusion Certain subgroups, such as smokers, patients in smaller practices, and physicians not yet considering changing their approach to cancer screening, could be targeted in future intervention studies designed to provide preventive services in primary care settings.
INTRODUCTION
MANY NATIONAL organizations have recommended screening procedures to detect cancer in its earlier stages.1-3 Nevertheless, the percentage of physicians who comply with recommendations for the use of preventive services remains low. In a study conducted by the American Cancer Society,2 less than 50% of physicians actually meet or exceed such recommendations. Other studies show that physicians frequently overestimate their actual compliance with screening guidelines.4 In the primary care setting, physicians who fail to follow screening recommendations do so for various reasons. These include the failure to remember to recommend screening, concerns about the cost to patients, and competing demands of primary care, including those of acute care and chronic illnesses.5-6 There is evidence to suggest that patients desire more frequent cancer screening than they are receiving.7
Various mechanisms have been recommended to increase screening, including the use of flow sheets and other physician and patient reminders,8-9 as well as physician and patient education. Several studies10-11 have reported increases in preventive services when interventions target entire offices by involving office staff such as physician assistants and nurses. Some authors12-15 recommend computerized reminder systems to overcome the forgetfulness of busy physicians and patients. Nevertheless, the integration of preventive services into practices has been slow.9, 16
Approaches to providing preventive services vary among primary care practices. Some practices offer a screening examination when a person presents to a physician for another reason, thus attempting to reduce the number of missed opportunities for screening. The United States Preventive Services Task Force (USPSTF) has recommended using routine visits as the opportunity for screening. Opportunistic screening, however, compared with visits scheduled specifically for screening, has poor coverage and may identify a disproportionate number of persons with comorbid illness, which may give the search for cancer or another disease a lower priority. For these reasons, opportunistic screening as the sole approach to cancer screening has been considered ineffective.17
A few studies have suggested that a scheduled health maintenance visit (HMV) may present an ideal opportunity for screening asymptomatic persons. In an intervention study to increase compliance with mammography recommendations through the use of health assessment forms,18 the rate of mammogram completion was greater in women who made an HMV than that in those who did not make an HMV (65% vs 21%). This study was limited to 2 practices, however, a study practice and a control practice. A previous study of cancer screening conducted in Colorado evaluated the effectiveness of various interventions on the delivery of cancer prevention services, targeting screening for breast, cervical, prostate, and skin cancer. The effects of the interventions were modest. The study found, however, that cancer screening was more likely to occur if the patient had an HMV in the previous year.19 Table 1 reproduces these findings and shows, for patients who had an HMV and for those who did not, the percentages current for cancer screening according to National Cancer Institute guidelines. Because of the importance of HMVs and because of their strong association with cancer screening, this study analyzes the medical records of 5746 patients to identify the practice, physician, and patient characteristics predictive of whether a patient had an HMV in the previous year.
|
|

|
|
Table 1. Patients Who Had Cancer Screening According to Whether They Had a Health Maintenance Visit (HMV) (N = 5746)*
|
|
|
SUBJECTS AND METHODS
As part of a randomized controlled trial targeted at increasing cancer screening, 5746 patient medical records were examined at baseline from 132 nonacademic general internists and family physicians throughout Colorado from June through November 1992. Study participants were members of the Copic Insurance Company, Greenwod Village, Colo, a physician-owned medical liability insurer that represents more than 80% of all physicians in private practice in Colorado.
The 132 physicians were selected as follows: A questionnaire that measured physicians' readiness to change behavior to improve disease prevention was sent to all 1041 primary care physicians in Colorado enrolled in the Copic Insurance Company.20 Readiness scores were calculated for 745 physicians (71.6% response rate) from 470 practices who returned the questionnaire. There were 411 practices in which at least two thirds of the physicians were insured by the Copic Insurance Company, and at least half of the physicians returned the questionnaire. These were classified as either rural or urban practices and ranked according to average readiness scores within rural or urban strata. One physician was chosen to represent each practice, ie, the sentinel physician, the most-ready physician from the most-ready practices, and the least-ready physician from the least-ready practices. With a goal of recruiting 32 physicians from each of 4 strata, we were able to recruit 132 (68.4%) of 193 sentinel physicians contacted: 32 in each of the ready rural and nonready urban strata, 31 in the ready urban strata, 28 in the nonready rural strata, and 9 from additional practices as part of a secondary study examining the possible effects of a baseline assessment. The last were recruited from the pool that fell between the most- and least-ready urban practices because the rural practices were nearly depleted. The sampling design ensured representation of both rural and urban practices and provided adequate power to detect differences between ready and nonready physicians.
Approximately 22 female and 22 male patient medical records were randomly selected from each practice. To be eligible, a patient had to be between the ages of 42 and 74 years, be seen by the sentinel physician for more than half the office visits during the past 2 years, have made an office visit within the past 365 days, and have had an office visit before that time. Patients were ineligible if they had any cancer diagnosis, with the exception of nonmelanoma skin cancer. For each practice, 10% of the medical records sampled were reviewed twice to establish interrater agreement.
Physician readiness is based on Prochaska and DiClemente's transtheoretical model21 for readiness to change adopted and applied to physicians.20, 22 Physician readiness scores are based on self-reported current behaviors of cancer screening and counseling and intent to change those behaviors. Counseling items comprised questions about breast self-examination, smoking, diet, and skin protection. Screening items comprised questions about mammography, Papanicolaou test or pelvic examination, digital rectal examination, sigmoidoscopy, palpation of testicles, clinical skin examination, and oral cavity examinations. For each of the 11 items, physicians considered in the precontemplation stage (not thinking about changing a behavior) received a score of 1; those in the contemplation stage (planning to make a change within the next 6 months), a score of 2; those in the preparation stage (planning to make a change within the next month), a score of 3; and those in the action (recently initiated the change) or maintenance stage (sustaining that change for at least 6 months), a score of 4. Physicians' level of readiness was determined according to the mean score of their 11 questions: precontemplation: 1.0 to 1.75; contemplation: 1.76 to 2.50; preparation: 2.51 to 3.25; and maintenance: 3.26 to 4.0.
We examined the relationship of a patient's sex, age, and insurance and smoking status with whether the patient had an HMV in the previous year. Because there was some variation among practices as to what constituted an HMV, each physician was asked to provide the record reviewers with an operational definition, which was then used for that physician's practice in abstracting medical records. The 2 most common definitions were a complete physical examination or a visit specifically scheduled with asymptomatic patients for comprehensive screening and health education. The ethnicity of patients was not recorded.
Practice characteristics included urban vs rural setting and practice size by the number of full-time physicians in the practice. Physician characteristics included the physician's sex, age, specialty (family medicine vs internal medicine), the number of patients seen per day, and readiness to change cancer screening behaviors. The ethnicity of physicians was not considered, except to note that all but 8 physicians were white.
Whether or not a patient had an HMV in the previous year was modeled as a function of patient, physician, and practice characteristics using a logistic regression model. A final model involving a subset of predictors was determined using a backward selection procedure, with statistical significance defined at P=.05. Odds ratio estimates and their 95% confidence intervals were obtained from this model using the generalized estimating equations procedure to account for the correlation among patients within a practice.23
RESULTS
Summary statistics for patients are shown in Table 2. The mean age was 55.4 years for male patients and 55.7 years for female patients. Table 2 shows that 86% had private insurance, whereas 4.5% paid for health care out of their own pockets, 2.6% used Medicaid, and the insurance status could not be determined or was not recorded in the medical record for 6.9% of the patients. Furthermore, 21.6% of patients were smokers, and 56.5% were between the ages of 50 and 69 years, considered to be the middle years for this sample.
|
|

|
|
Table 2. Distribution of Patient Characteristics and Percentage of Patients Who Had a Health Maintenance Visit (HMV) Scheduled in the Previous Year
|
|
|
The distributions of practice and physician characteristics are given in Table 3. Our sample of 132 physicians had a higher proportion of family physicians than the population of 1041 physicians enrolled in the Copic Insurance Company from which they were drawn (76.5% vs 69%) because we oversampled rural practices, which tended to have a higher proportion of family physicians than urban practices. In our sample, physicians ranged in age from 30 to 74 years, with a mean of 45.8 years, and 18 (13.6%) physicians were women. The 1041 physicians had an average age of 46.5 years, and 9.7% were women. Sixty (45.4%) of the 132 physicians practiced in a rural location. Except for 1 practice that had 8 full-time physicians, practice sizes ranged from 0 to 5 full-time physicians, with an average of 1.6. Physicians saw an average of 24 patients a day, with a range of 8 to 60. Fifty-eight physicians (43.9%) were classified as being in the precontemplation stage of readiness.
|
|

|
|
Table 3. Distribution of Practice and Physician (MD) (N = 132) Characteristics and Percentage Who Scheduled a Health Maintenance Visit (HMV)
|
|
|
Female and male physicians did not differ significantly in specialty or location of practice (Table 4). General internists, however, were more likely to be in urban practices than family physicians (71% vs 49.5%; P =.04). Female physicians tended to be younger (39.4 vs 46.8 years; P=.01), indicated a greater willingness to change approaches to cancer screening (P=.06, based on mean readiness score), and saw fewer patients per day (21 vs 25; P=.04) than male physicians.
|
|

|
|
Table 4. Characteristics of Male and Female Physicians (MDs) (N = 132)
|
|
|
Overall, 31% of patients had an HMV. Table 2 gives the proportion of patients who had an HMV among different subgroups. In univariate analyses, women receive significantly more HMVs than men in age groups 42 to 49 years, 50 to 59 years, and 60 to 69 years (P<.05), but there was no significant difference between men and women aged 70 to 74 years (P=.44). Figure 1 presents a smooth fit of the percentage of patients with an HMV as a function of age, illustrating that differences between sexes depend on age.
|
|

|
|
Smoothed fit of health maintenance visit (HMV) by patient age.
|
|
|
Practice and physician characteristics in univariate analyses were associated with HMVs as well (Table 3). On average, female physicians provided HMVs to 38.2% of their patients compared with an average of 30% for male physicians (P=.01). Physicians in the precontemplation stage of readiness provided only 27% of their patients an HMV in the previous year compared with 33% to 36% of physicians in higher stages of readiness. The number of patients seen per day by a physician had a negative correlation with the percentage of patients having an HMV (r= -0.16, P =.06). The HMV was not significantly related to physician age, specialty, location of practice, or the number of full-time physicians.
Model adjusted odds ratios and their corresponding 95% confidence intervals are provided in Table 5 for statistically significant predictors in the final model. The interaction between patient age and sex shown in Figure 1 was modeled using age and sex categories, with men aged 50 to 69 as the reference category. Initial models fit separate effects for patients aged 50 to 59 and 60 to 69, but because these 2 age groups did not differ significantly for either sex, they were combined in the final model. Not surprisingly, we found for both sexes that patients aged 50 to 69 were significantly more likely to receive HMVs than patients aged 42 to 49 (P<.05). Male patients aged 50 to 69 did not differ significantly from male patients aged 70 to 74, whereas female patients aged 50 to 69 had 1.33 times higher odds of having an HMV than female patients aged 70 to 74 (P=.06). Within the age groups 42 to 49 and 50 to 69, female patients were significantly more likely to have HMVs than male patients (P<.05), but there were no significant differences between sexes among 70- to 74-year-old patients. Nonsmokers had 1.27 times higher odds of having an HMV than smokers (P<.001).
|
|

|
|
Table 5. Model Adjusted Odds Ratios for Health Maintenance Visit
|
|
|
Female physicians had 1.48 times higher odds of providing an HMV than male physicians (P=.01). Physicians in practices with 3 or more full-time physicians had 1.34 times higher odds of providing an HMV than physicians in smaller practices (P=.04). Physicians who were contemplating, preparing, or implementing changes in their cancer screening behaviors had 1.33 times higher odds of providing an HMV than physicians not yet thinking about making such changes (P=.03).
COMMENT
This study focused on the HMV as an important vehicle for the delivery of preventive services in a population of Colorado physicians. Because of a strong association of cancer screening with the presence of an HMV, we examined the predictors of the latter. An analysis was presented that described the characteristics of patients who were more likely to have an HMV, as well as the characteristics of practices and physicians more likely to provide HMVs.
Our study found that less than a third of patients aged 42 to 74 years have annual HMVs, despite recommendations for a higher level of preventive services. The USPSTF has recommended that every opportunity be taken to deliver preventive services; yet, our results suggest that these recommendations are not being broadly followed. As part of the physician readiness survey conducted in this study, physicians were asked to what degree—"not at all," "only a little," "moderately," or "strongly"—they were influenced by various organizations. Only 39 (30%) physicians cited the USPSTF as "moderately" or "strongly" influential compared with the American Cancer Society (90%), the Copic Insurance Company (77%), the National Cancer Institute (62%), and the American Medical Association (46%).
The finding that female physicians were more likely to provide HMVs than male physicians is supported by multivariate analyses that controlled for patient age and sex. Moreover, the difference in physician sex is not explained by differences in physician age. When considering only physicians aged 50 years or younger, the 18 female physicians provide significantly more HMVs (38.2%) than the 79 male physicians (30.4%). These results are consistent with other studies that have found that female physicians provide more screening than male physicians for breast and cervical cancers.24
The study found that the use of HMV was greater in practices that had 3 or more full-time physicians than in those with 2 or fewer full-time physicians, suggesting that the greater resources may have a positive effect on the delivery of preventive services. A possible explanation is that larger offices tend to have office systems that encourage screening.
The use of HMV was positively associated with physician readiness to change approaches to cancer screening, implying that HMV is an important mediating mechanism for the delivery of preventive services for these physicians. Not surprisingly, physicians in the action or maintenance stage of readiness, ie, those physicians already performing cancer screening, were more likely to give an HMV than physicians in the precontemplation stage. In addition, physicians planning to make a change in screening or counseling practices were more likely to provide HMVs than physicians not thinking about changing behaviors.
Predictors of HMVs included patient characteristics as well. Because most screening recommendations start at age 50 years, it is not surprising that patients between 50 and 69 years old received more HMVs than patients aged 42 to 49 years. The lower rate of HMV for women aged 70 to 74 years compared with women aged 50 to 69 years might be explained by demands of competing comorbidity and a lower priority given to screening by physicians and patients. The rate of HMV for men aged 70 to 74 years, however, did not differ significantly from that of men aged 50 to 69 years. Interestingly, the higher rate of HMV for women than men for patients aged 69 years and younger did not exist for patients aged 70 years and older. The rather stable levels of HMVs for men older than 50 years is perhaps explained by the influence on this group of physicians of the American Cancer Society's endorsement of annual screening for prostate cancer for men in this age group.25 Strong associations in these data between HMVs and prostate cancer screening (see "Digital rectal examination" in Table 1) support this contention. The USPSTF recommended against prostate cancer screening interventions and was cited as 1 of the least influential organizations by the group of Colorado physicians studied.
The study also found that smokers are less likely to have HMVs; yet, they are more at risk for cancer than nonsmoking patients. A possible explanation is that nonsmokers are more health conscious than smokers. Another possible explanation is that physicians are less likely to provide HMVs to patients engaging in higher risk behaviors such as smoking.
Financial barriers also limit patients' accessibility to preventive care. Changes in our health care system toward managed care, capitation, and copayments may determine the types of ambulatory visits considered desirable by patients, physicians, and health care administrators. An HMV, which may take twice as long as a typical visit for an acute condition and involve some costly screening examinations, may not be viewed by physicians as a cost-effective investment of time and resources. Because most cancer screening occurs in the context of an HMV, this study suggests that increased levels of reimbursement by health maintenance organizations for HMVs would encourage higher levels of cancer screening.
The study has several limitations. First, there was some variation among physicians in the definition of an HMV. A possible effect of such excess variation is reduced statistical power or a greater possibility of the failure to detect real differences. Second, the study did not assess patients' attitudes toward HMVs, and it did not examine patients' and physicians' knowledge of cancer screening. Finally, we were not able to conclude with reasonable statistical certainty that patients with Medicaid had fewer HMVs because more than 92.4% of patients for whom insurance status was known had private insurance.
CONCLUSIONS
This study of Colorado primary care practices suggests that the use of the HMV varies among subgroups of patients and physicians. As expounded by the USPSTF, improvement in cancer screening requires that physicians take every opportunity to screen. Abstracting patients' medical records revealed, however, that most screening occurred in the context of an HMV. This disparity may be partly explained by the reported minimal influence, relative to other national organizations, of the USPSTF on physicians in our study. Given that the cost-effectiveness of various screening examinations will remain a central issue in determining the delivery of preventive services,26 a directed approach involving identifying appropriate subgroups for screening is needed. Data on Colorado physicians and their patients suggest that the consideration of patient, physician, and practice characteristics in promoting the use of HMVs may offer an effective strategy in moving toward the recommended screening guidelines.
AUTHOR INFORMATION
Accepted for publication September 9, 1997.
This study was supported by research grant HS 06992 from the Agency for Health Care Policy and Research, Rockville, Md.
Reprints: John S. Preisser, PhD, Department of Public Health Sciences, Wake Forest University School of Medicine, Medical Center Boulevard, Winston-Salem, NC 27157-1063 (e-mail: jpreisse{at}rc.phs.wfubmc.edu).
From the Health Services Research Center, Department of Public Health Sciences (Drs Preisser, Cohen, Moran, and Shelton), and the Department of Internal Medicine (Drs Cohen, Wofford, and Moran), Wake Forest University School of Medicine, Winston-Salem, NC; and the Division of Biostatistics, AMC Cancer Research Center (Drs Cohen and McClatchey and Ms Wolfe), Denver, Colo. Dr McClatchey is now with the Kempe Prevention Research Center for Family and Child Health, Denver.
REFERENCES
1. Smart CR. Working Guidelines for Early Cancer Detection: Rationale and Supporting Evidence to Decrease Mortality. Bethesda, Md: National Cancer Institute, Early Detection Branch, Division of Cancer Prevention and Control; 1987.
2. American Cancer Society. Survey of physicians' attitudes and practices in early cancer detection. CA Cancer J Clin. 1985;35:197-213.
WEB OF SCIENCE
| PUBMED
3. US Preventive Services Task Force. Guide to Clinical Preventive Services: Report of the US Preventive Services Task Force. Baltimore, Md: Williams & Wilkins; 1996.
4. Lurie N, Manning WG, Peterson C, Goldberg GA, Phelps CA, Lillard L. Preventive care: do we practice what we preach? Am J Public Health. 1986;76:1009-1013.
WEB OF SCIENCE
| PUBMED
5. Frame PS, Werth PL. How primary health care providers can integrate cancer prevention into practice. Cancer. 1993;72(suppl):1132-1137.
6. Jaén CR, Stange KC, Nutting PA. Competing demands of primary care: a model for the delivery of clinical preventive services. J Fam Pract. 1994;38:166-171.
WEB OF SCIENCE
| PUBMED
7. Woo B, Woo B, Cook EF, Weisberg M, Goldman L. Screening procedures in the asymptomatic adult: comparison of physicians' recommendations, patients' desires published guidelines, and actual practice. JAMA. 1985;254:1480-1484.
FREE FULL TEXT
8. Gann P, Melville SK, Luckmann R. Characteristics of primary care office systems as predictors of mammography utilization. Ann Intern Med. 1993;118:893-898.
FREE FULL TEXT
9. Melville SK, Luckmann R, Coghlin J, Gann P. Office systems for promoting screening mammography: a survey of primary care practices. J Fam Pract. 1993;37:569-574.
WEB OF SCIENCE
| PUBMED
10. Davidson RA, Fletcher SW, Retchin S, Duh S. A nurse-initiated reminder system for the periodic health examination: implementation and evaluation. Arch Intern Med. 1984;144:2167-2170.
FREE FULL TEXT
11. McPhee SJ, Detmer WM. Office-based interventions to improve delivery of cancer prevention services by primary care physicians. Cancer. 1993;72(suppl):1100-1112.
12. McDonald CJ, Hui SL, Smith DM, et al. Reminders to physicians from an introspective computer medical record: a two-year randomized trial. Ann Intern Med. 1984;100:130-138.
13. McPhee SJ. Computer-assisted preventive care. Arch Fam Med. 1994;3:576-578.
FREE FULL TEXT
14. Frame PS, Zimmer JG, Werth PL, Hall WJ, Eberly SW. Computer-based vs manual health maintenance tracking: a controlled trial. Arch Fam Med. 1994;3:581-588.
FREE FULL TEXT
15. Moran WP, Wofford JL, Hamrick V, et al. Implementing computerized tracking at a community health center: challenges and solution. Proc Annu Symp Comput Appl Med Care. Fall 1993:139-143.
16. McGinnis JM, Griffith HM. Put prevention into practice: a systematic approach to the delivery of clinical preventive services. Arch Intern Med. 1996;156:130-132.
FREE FULL TEXT
17. Law M. "Opportunistic" screening. J Med Screening. 1995;1:208.
18. Yarnell SH, Michener JL, Broadhead WE, Tse CKJ. Increasing compliance with mammography recommendations: health assessment forms. J Fam Pract. 1993;36:59-64.
WEB OF SCIENCE
| PUBMED
19. Cohen SJ, McClatchey MW, Wolfe P, Shelton BJ, Archer PG. Health maintenance visit as the determinant of cancer screening by primary care physicians [abstract]. J Gen Intern Med. 1996;11S(suppl):121.
20. Cohen SJ, Halvorson HW, Gosselink CA. Changing physician behavior to improve disease prevention. Prev Med. 1994;23:284-291.
FULL TEXT
|
WEB OF SCIENCE
| PUBMED
21. Prochaska JO, DiClemente CC. Transtheoretical therapy: toward a more integrative model of change. Psychother Theory Res Pract. 1982;19:276-288.
FULL TEXT
22. Main DS, Cohen SJ, DiClemente CC. Measuring physician readiness to change cancer screening: preliminary results. Am J Prev Med. 1995;11:54-58.
WEB OF SCIENCE
| PUBMED
23. Liang KY, Zeger SL. Regression analysis for correlated data. Annu Rev Public Health. 1993;14:43-68.
FULL TEXT
|
WEB OF SCIENCE
| PUBMED
24. Lurie N, Margolis KL, McGovern PG, Mink PJ, Slater JS. Why do patients of female physicians have higher rates of breast and cervical cancer screening? J Gen Intern Med. 1997;12:34-43.
FULL TEXT
|
WEB OF SCIENCE
| PUBMED
25. Metlin C, Jones G, Averette H, Gusberg SB, Murphy GP. Defining and updating the American Cancer Society guidelines for the cancer-related check-up: prostate and endometrial cancers. CA Cancer J Clin. 1993;43:42-46.
WEB OF SCIENCE
| PUBMED
26. Battista RN, Grover SA. Early detection of cancer: an overview. Annu Rev Public Health. 1988;9:21-45.
FULL TEXT
|
WEB OF SCIENCE
| PUBMED
RELATED ARTICLES
Cancer Screening: A Challenge in Today's Changing Practice of Medicine
Roselyn Payne Epps
Arch Fam Med. 1998;7(4):315-316.
FULL TEXT
Can We Change Physicians' Practices in the Delivery of Cancer-Preventive Services?
Mack T. Ruffin IV
Arch Fam Med. 1998;7(4):317-319.
FULL TEXT
Cancer Early-Detection Services in Community Health Centers for the Underserved: A Randomized Controlled Trial
Allen J. Dietrich, Jonathan N. Tobin, Carol Hill Sox, Andrea N. Cassels, Fredeswinda Negron, Richard G. Younge, Neal A. Demby, and Tor D. Tosteson
Arch Fam Med. 1998;7(4):320-327.
ABSTRACT
| FULL TEXT
Prescribe for Health: Improving Cancer Screening in Physician Practices Serving Low-Income and Minority Populations
Clara Manfredi, Ronald Czaja, Sally Freels, Mitchell Trubitt, Richard Warnecke, and Loretta Lacey
Arch Fam Med. 1998;7(4):329-337.
ABSTRACT
| FULL TEXT
THIS ARTICLE HAS BEEN CITED BY OTHER ARTICLES
 |
Themed Review: Clinical Interventions to Promote Physical Activity in Youth
Meriwether et al.
AMERICAN JOURNAL OF LIFESTYLE MEDICINE 2008;2:7-25.
ABSTRACT
Delivery of Cancer Screening: How Important Is the Preventive Health Examination?
Fenton et al.
Arch Intern Med 2007;167:580-585.
ABSTRACT
| FULL TEXT
Use of Annual Physical Examinations by Aging Chinese Canadians
Lai and Kalyniak
J Aging Health 2005;17:573-591.
ABSTRACT
Improving Quality Improvement Using Achievable Benchmarks For Physician Feedback: A Randomized Controlled Trial
Kiefe et al.
JAMA 2001;285:2871-2879.
ABSTRACT
| FULL TEXT
Reminders for Health Maintenance Visits
Cohen et al.
Arch Fam Med 1999;8:295-296.
FULL TEXT
Can we change physicians' practices in the delivery of cancer-preventive services?
Epps
Arch Fam Med 1998;7:315-316.
FULL TEXT
Can We Change Physicians' Practices in the Delivery of Cancer-Preventive Services?
Ruffin IV
Arch Fam Med 1998;7:317-319.
FULL TEXT
|