 |
 |

Rofecoxib, a New Cyclooxygenase 2 Inhibitor, Shows Sustained Efficacy, Comparable With Other Nonsteroidal Anti-inflammatory Drugs
A 6-Week and a 1-Year Trial in Patients With Osteoarthritis
Kenneth Saag, MD, MSc;
Desiree van der Heijde, MD;
Chester Fisher, MD;
Adil Samara, MD;
Lisa DeTora, PhD;
James Bolognese, MS;
Rhoda Sperling, MD;
Brian Daniels, MD;
for the Osteoarthritis Studies Group
Arch Fam Med. 2000;9:1124-1134.
ABSTRACT
 |  |
Introduction Rofecoxib, a cyclooxygenase 2 inhibitor (sometimes known as a specific cyclooxygenase 2 inhibitor or Coxib), is used in osteoarthritis (OA). Published information indicates rofecoxib's improved gastrointestinal safety profile over nonselective nonsteroidal anti-inflammatory agents (NSAIDs).
Objective To evaluate the efficacy and safety of rofecoxib in treating OA in 2 studies.
Methods Two randomized, double-blind, parallel-group studies in patients with OA of the knee or hip were conducted using identical entry criteria and end points. A 6-week placebo-controlled trial in 736 patients compared 12.5 and 25 mg of rofecoxib once daily with 800 mg of ibuprofen 3 times daily, and a 1-year study compared 12.5 and 25 mg of rofecoxib once daily with 50 mg of diclofenac 3 times daily in 693 patients.
Results Rofecoxib, at 12.5 and 25 mg, demonstrated efficacy clinically comparable with ibuprofen, assessed by 3 primary end points according to predefined comparability criteria. Both rofecoxib doses and ibuprofen provided significantly greater efficacy than placebo on all primary end points at 6 weeks. Both rofecoxib doses and diclofenac showed similar efficacy over 1 year. All treatments were well tolerated.
Conclusions Rofecoxib is effective in treating OA with once-daily dosing for 6 weeks and 1 year. Rofecoxib was generally safe and well-tolerated in OA patients for 6 weeks and 1 year.
INTRODUCTION
OSTEOARTHRITIS (OA) is the most common joint disorder worldwide, affecting between 15 and 30 million people in the United States alone. It is the leading cause of activity limitation in women older than 45 years1 and second to ischemic heart disease as a cause of disability in men older than 50 years.2 Most patients with OA are treated, at least initially, by primary care physicians.3
Osteoarthritis is a chronic disease characterized by joint pain, stiffness, and loss of physical function.4-5 Its onset is age-associated, usually between ages 50 and 60 years. Abnormal joint morphology, prior joint injury, and obesity can all increase the risk for developing OA, particularly in weight-bearing joints (eg, hip or knee).6-13 Patients generally require long-term treatment for their OA symptoms, especially joint pain and stiffness.13-16 Although pure analgesics, such as acetaminophen, are considered a first-line therapy in some osteoarthritis guidelines, nonsteroidal anti-inflammatory drugs (NSAIDs) are the most commonly used class of medications for treating OA pain and stiffness because of their analgesic and anti-inflammatory effects.15-17
Nonsteroidal anti-inflammatory drug use is associated with dyspeptic symptoms (including nausea, heartburn, and abdominal pain) and gastroduodenal mucosal injury (including endoscopically or clinically diagnosable ulceration or erosions). The most clinically significant mucosal injuries, perforations, ulcers, or bleeding, result in at least 100 000 hospitalizations and 10 000 deaths annually in the United States.18-19 Correlation between dyspeptic symptoms and mucosal injury is poor in patients taking NSAIDs.20 Incurred health care costs from gastropathies are compounded by associated work and productivity loss, and represent an important public health issue.
Nonsteroidal anti-inflammatory drugs inhibit cyclooxygenase (COX), a mediator of prostaglandin synthesis. Two distinct isoforms, COX-1 and COX-2, have been identified. Traditional NSAIDs are dual COX-1/COX-2 inhibitors.21-22 It was posited that selective COX-2 inhibition would provide comparable efficacy for the treatment of painful or inflammatory conditions, presumed to be mediated by COX-2, without the gastropathy associated with COX-1 inhibition.
The trials described examined the efficacy and safety of rofecoxib, a COX-2 inhibitor, in treating the signs and symptoms of OA, compared with placebo and NSAID comparators. One study provided placebo and active comparator-controlled data for 6 weeks and the other, active-comparator controlled data for 1 year. These studies employed validated end points that measure efficacy on a core set of OA characteristics identified by Outcomes Measures in Rheumatoid Arthritis Clinical Trials (OMERACT), an international consensus group of rheumatologists.23-24 The primary efficacy hypothesis was that rofecoxib would demonstrate comparable efficacy to active NSAID comparators according to strict predefined criteria.
PATIENTS AND METHODS
PROTECTION OF PATIENTS
Both studies were conducted in accordance with ethical considerations for the protection of patients, as outlined in the Declaration of Helsinki, and were approved by appropriate institutional review boards or ethical review committees. All patients gave written, informed consent before undergoing any examination or study procedure.
PATIENTS AND STUDY POPULATION
In both studies, men and women 40 years and older with OA of the knee or hip were eligible to enroll. All patients met clinical and radiographic criteria for OA, and were American Rheumatism Association functional classes I, II, or III.25 Patients had a history of positive therapeutic benefit from NSAIDs (90% of patients) or acetaminophen (10% of patients).
Patients were excluded from study participation if they were using corticosteroids, topical analgesics, low-dose aspirin, regular antacid, H2 blocker, proton pump inhibitors, warfarin, or ticlopidine. They were also not allowed to enroll if they had significant renal impairment (<30 mL/min calculated creatinine clearance), evidence of active gastrointestinal (GI) bleeding, GI malabsorption syndrome, class III/IV angina or congestive heart failure, uncontrolled hypertension, stroke, transient ischemic attack (within 2 years), active hepatic disease, recent neoplastic disease, allergy to acetaminophen or NSAIDs, and any other condition that, in the opinion of the investigator, could interfere with study participation, confound study results, or pose an unacceptable risk to the patient.
STUDY DESIGN
The 6-week ibuprofen study was a randomized, placebo and active comparator controlled trial, comparing placebo, rofecoxib, 12.5 and 25 mg (once daily in the morning), and ibuprofen, 2400 mg (800 mg 3 times daily) in patients with OA of the knee or hip. Patients were randomized by a computer-generated allocation schedule to 1 of 4 arms: placebo, rofecoxib, 12.5 and 25 mg, and ibuprofen. Patients were allocated to placebo and active treatments (rofecoxib and ibuprofen) in a 1:4:4:4 scheme. Treatments were administered in blinded blister cards containing morning (rofecoxib, 12.5 mg or matching placebo, 25 mg or matching placebo, and active comparator or matching placebo) afternoon, and evening doses (active comparator or matching placebo). Patients took 3 tablets each morning and 1 tablet each afternoon and evening.
Eligible patients who used a prestudy NSAID had to demonstrate a worsening in the signs and symptoms of OA after a washout period. Eligible patients who used prestudy acetaminophen were required to have consistently at least moderate symptoms of OA.
Patients returned for evaluation after 2, 4, and 6 weeks of study therapy or at the time of a premature discontinuation. Patients underwent a poststudy safety assessment, including physical examination and laboratory tests 7 to 10 days after completing or discontinuing from the trial. During the treatment period, patients were provided with acetaminophen tablets (325 mg) as rescue medication for breakthrough OA pain. Acetaminophen use (number of tablets) was recorded at each visit.
All primary and key secondary end points were reported using well-validated methods for evaluating the clinical efficacy of OA therapies, as explained below. Most end points were measured on a 0- to 4-point Likert scale (Likert) or 100-mm visual analog scale (VAS). Several derive from the Western Ontario and McMaster Universities (WOMAC) Osteoarthritis Index questionnaire.23
The 1-year diclofenac study employed identical study procedures to the 6-week ibuprofen study. However, the 1-year study compared rofecoxib, 12.5 and 25 mg (once daily in the morning) with diclofenac, 150 mg (50 mg 3 times daily) for 1 year. Patients were allocated 1:1:1 to rofecoxib, 12.5 or 25 mg, or diclofenac. Patients returned for efficacy evaluations after 2, 4, 8, 12, 24, 39, and 52 weeks of therapy and discontinuation. Only after the first 26 weeks of treatment, topical or systemic analgesics and corticosteroids were permitted for breakthrough OA pain.
In both studies, investigators assessed treatment compliance based on returns and counts of all blister cards dispensed, and tablet counts of rescue acetaminophen.
EFFICACY ASSESSMENTS
Outcome assessments were the same for both trials. An individual patient was examined by the same researcher at all study visits.
The primary end points were pain walking on a flat surface (WOMAC pain subscale question 1; 100-mm VAS [0 mm, no pain 100 mm, extreme pain]), a patient global assessment of response to therapy (Likert scale of 0, none, to 4, excellent), and an investigator global assessment of disease status (Likert scale of 0, very poor, to 4, very well). Secondary end points included the WOMAC physical function, stiffness, and pain subscales (100-mm VAS [0, no difficulty, pain, or stiffness, to 100 mm, extreme difficulty, pain, or stiffness]); a patient global assessment of disease status (0 to 100-mm VAS; 0 mm, very poor 100 mm, very well); the percentage of patients discontinuing due to lack of efficacy; acetaminophen use for rescue (number of tablets per patient per day); an investigator global assessment of response to therapy (Likert scale of 0, none, to 4, excellent); and study joint tenderness (scale: 0, none, to 3, patient states pain, winces, and withdraws).
The WOMAC pain and stiffness subscales included questions about pain at night while in bed and stiffness on awakening.
SAFETY AND TOLERABILITY ASSESSMENTS
Safety assessments were made identically in both trials.
At each visit, a physical examination, including weight and vital signs, was performed and evaluated. Laboratory evaluations made at each visit included complete blood count, serum chemistry, and urinalysis evaluations. All adverse experiences were reported by the patient during a nondirected interview at each study visit. Each adverse experience was evaluated by the investigator as to its intensity (mild, moderate, or severe) and the relationship to study drug, either not drug-related (definitely not, probably not) or drug-related (possibly, probably, or definitely). The investigator made these assessments while blinded to study treatment. The outcome and action taken for each adverse experience were recorded. Statistical analyses were performed on all adverse experiences and on those adverse experiences categorized as drug-related.
STATISTICAL ANALYSIS
The response for efficacy end points was the change from baseline (the assessment immediately before randomization, after washout of prior therapy, NSAIDs, or acetaminophen). The analysis was performed using an analysis of covariance (ANCOVA) based on the intention-to-treat principle, but only included patients with a randomization, plus at least 1 treatment measurement, to avoid imputing missing data. The ANCOVA model included terms for treatment, study center, stratum, and baseline score as the covariate. Interactions with treatment were tested and removed from the model if nonsignificant at P>.05.
Two conditions had to be met for clinical comparability between treatments: in any 2 of the primary end points, the 95% confidence intervals (CIs) of mean differences between treatment groups were to be within predefined comparability bounds (±10 mm on a 100-mm VAS and ±0.5 on a Likert scale); and all 3 of the posterior probabilities (with noninformative prior distributions) that the true mean differences are within the predefined clinical comparability bounds were P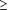 .95. These clinical comparability bounds are more conservative than those proposed by a consensus of academic rheumatologists24-26 and were derived from the results of previous OA trials with rofecoxib. The CIs and posterior probabilities were derived from appropriate summary statistics and from the respective ANCOVA, as were tests of difference from placebo. .95. These clinical comparability bounds are more conservative than those proposed by a consensus of academic rheumatologists24-26 and were derived from the results of previous OA trials with rofecoxib. The CIs and posterior probabilities were derived from appropriate summary statistics and from the respective ANCOVA, as were tests of difference from placebo.
In the 6-week ibuprofen study, the primary analysis was based on the average change from baseline over the 6-week treatment period. Pairwise treatment comparisons with rofecoxib were carried out in a step-down fashion. For difference from placebo, a step-down approach was used; the 25-mg dose was tested first, and only if it was significantly different was the 12.5-mg dose tested. For comparability with active control, only if the 95% CI for the difference from 25 mg was within the comparability bounds did we compare the 95% CI for 12.5 mg. The incidence of adverse experiences was compared among groups using Fisher exact test. All statistical tests were 2-tailed and values of P<.05 were considered significant.
The 6-week ibuprofen study was powered to detect differences of approximately 0.86 on the Likert scale and 14 mm on the VAS between rofecoxib and placebo with 99% power ( = .05, 2-tailed), given a sample size of 50 (placebo) and 200 patients (rofecoxib). There was at least 99% power to yield the 95% CIs within the comparability ranges for the 3 primary end points if the true difference between rofecoxib and ibuprofen would be 0, assuming 200 patients receiving each active treatment. = .05, 2-tailed), given a sample size of 50 (placebo) and 200 patients (rofecoxib). There was at least 99% power to yield the 95% CIs within the comparability ranges for the 3 primary end points if the true difference between rofecoxib and ibuprofen would be 0, assuming 200 patients receiving each active treatment.
The statistical analyses were similar for the 1-year study. In the 1-year diclofenac study, primary efficacy analyses were based on the average change from baseline over the first 12-week treatment period. Analyses were also performed at 26 and 52 weeks. A secondary analysis of the last observed value in each treatment period, and an analysis of patients who completed the study, were performed to confirm the findings of the primary analysis.
Post hoc analyses of 2 individual questions from the WOMAC pain and stiffness subscales, pain at night and stiffness on awakening in the morning, were performed as described above for the primary analyses.
Because of differences in study design (length of the study and controls), data from the 2 trials cannot be combined. These appear separately as the difference from placebo or the active comparators and the durable efficacy of rofecoxib in this population. The 1-year study was powered similarly to the 6-week study.
All comparisons between pairs of treatment groups were made using Fisher exact test.
RESULTS
PATIENTS
In the 6-week ibuprofen study, 1156 patients were screened and 736 (64%) enrolled in 62 clinical centers (including university hospitals, private practices, and independent research facilities) in the United States. Patients were not randomized after screening for 1 or more of the following reasons: failure to satisfy OA criteria (n = 257), medical exclusion (n = 190), failure to meet flare criteria after NSAID washout (n = 80), or use of prohibited medications (n = 61). Of the randomized patients, 74.5% were women. Age ranged from 39 to 91 years. Other baseline characteristics were comparable among treatment groups (Table 1).
|
|

|
|
Table 1. Baseline Patient Characteristics*
|
|
|
In the 1-year diclofenac study, 909 patients were screened and 693 (76%) enrolled in 43 clinical centers (including university hospitals, private practices, and independent research facilities) in 22 countries worldwide. Most commonly patients were not randomized after screening for 1 or more of the following reasons: medical condition (n = 128), failure to meet flare criteria after NSAID washout (n = 45), failure to satisfy OA criteria (n = 23), and the use of prohibited medications (n = 23). Of the randomized patients, 80.1% were women. Age ranged from 38 to 85 years (Table 1).
Important concurrent medical conditions in the 6-week ibuprofen study were as follows: 43% of patients had hypertension, 6% had diabetes, 46% had drug allergies, 27% had hypothyroidism, and 29% had hypercholesterolemia. In the 1-year diclofenac study, 34% of patients had a secondary diagnosis of hypertension, 5% had type 2 diabetes mellitus, 3% had drug allergies, 7% had hypercholesterolemia, and 5% had hypothyroidism.
EFFICACY
The majority of patients completed the full specified treatment in each study (Table 2): 84.9% completed the 6-week ibuprofen study, and 66.5% of patients completed the 1-year diclofenac study. Fewer than 1% of patients were missing sufficient data (either the baseline or all treatment period values) to exclude them from the efficacy analyses.
|
|

|
|
Table 2. Patient Accounting*
|
|
|
6-WEEK IBUPROFEN STUDY
For all WOMAC scales, patient and investigator global assessments, all 95% CIs for difference between each rofecoxib dose and diclofenac were contained within the prespecified comparability bounds (posterior probability >.999). These changes were sustained over the 6-week period. In the 6-week ibuprofen study, those receiving 25 and 12.5 mg of rofecoxib showed improvement superior to placebo (P<.001) and comparable with ibuprofen for the WOMAC pain (P = .21 and P = .64, respectively), physical function (P = .11 and P = .93, respectively), and stiffness subscales (P = .28 and P = .91, respectively) over the 6-week treatment period. These effects were seen at the first measurement of efficacy after starting study drug (2 weeks) and sustained throughout the treatment period (Figure 1).
|
|

|
|
Figure 1. Mean change plots of the primary efficacy end points in the 6-week ibuprofen study. S indicates screening visit; R, randomization visit/baseline assessment.
|
|
|
Results were consistent for patient global assessments of response to therapy and the patient global assessment of disease status, compared with placebo (P<.001) or ibuprofen (P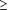 .16). The investigator global assessment of disease status and the investigator global assessment of response to therapy (Likert scale) also gave similar results for rofecoxib compared with placebo (P<.001) and ibuprofen (P .16). The investigator global assessment of disease status and the investigator global assessment of response to therapy (Likert scale) also gave similar results for rofecoxib compared with placebo (P<.001) and ibuprofen (P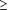 .25) (Table 3). These effects were seen at the first measurement of efficacy after administration of the study drug (2 weeks) and sustained throughout the 6-week treatment period. .25) (Table 3). These effects were seen at the first measurement of efficacy after administration of the study drug (2 weeks) and sustained throughout the 6-week treatment period.
|
|

|
|
Table 3. Efficacy End Points, Difference From Baseline*
|
|
|
Patients receiving 25 or 12.5 mg of rofecoxib discontinued because of lack of efficacy less frequently compared with patients receiving placebo (P<.001 and P = .01, respectively) (Table 2). Patients receiving placebo used significantly more acetaminophen tablets per day (1.49 tablets; P<.001, vs rofecoxib and ibuprofen) than patients receiving rofecoxib (1.05 tablets) or ibuprofen (0.93 tablets). Patients receiving rofecoxib at 12.5 or 25 mg, or ibuprofen had significantly less tenderness in their primary study joints than patients receiving placebo (P<.001) (Table 3).
Patients taking 12.5 or 25 mg of rofecoxib once daily, or ibuprofen 3 times daily, had significant decreases in stiffness on awakening and pain at night while in bed (Figure 2).
|
|

|
|
Figure 2. Mean change plots for individual Western Ontario and McMaster Universities Osteoarthritis Index questions (WOMAC). S indicates screening visit; R, randomization visit/baseline assessment.
|
|
|
The patient global assessment of response to therapy showed that more than 80% of patients who received rofecoxib at 12.5 mg (83%), 25 mg (86%), or ibuprofen (81%) demonstrated a clinical response of at least fair (P<.001 vs placebo) (Figure 3).
|
|

|
|
Figure 3. Percentage of patients who reported treatment effect (fair, good, or excellent) by treatment group, for the 6-week ibuprofen study.
|
|
|
1-YEAR DICLOFENAC STUDY
The 95% CIs for the difference between 12.5 and 25 mg of rofecoxib vs diclofenac were contained within the prespecified comparability bounds for the WOMAC scales (posterior probability >.999), indicating clinical comparability of responses among the 3 treatments. In the 1-year diclofenac study, 25 mg of rofecoxib showed marked improvement from baseline and comparable efficacy with diclofenac on the WOMAC physical function (P = .07), stiffness (P = .15), and pain subscales (P = .11) over the 1-year treatment period. Rofecoxib at 12.5 mg was significantly different from diclofenac (P<.05), but comparable with 25 mg, with marked improvement from baseline for the 3 subscales. Similar results were seen at 2, 12, 26, and 52 weeks (Figure 4).
|
|

|
|
Figure 4. Mean change plots of the primary efficacy end points in the 1-year diclofenac study. S indicates screening visit; R, randomization visit/baseline assessment.
|
|
|
The 95% CIs for the difference between 12.5 and 25 mg of rofecoxib vs diclofenac were contained within the prespecified comparability bounds for all of the global assessments (posterior probability >.999), indicating clinical comparability of responses among the 3 treatments. Efficacy results were consistent for each dose of rofecoxib compared with diclofenac for the end points: patient global assessment of response to therapy and investigator global assessment of disease status (P = .03 vs 25 mg, and P = .01 vs 12.5 mg, respectively). For patient global assessment of disease status, rofecoxib was different from diclofenac (P = .01). For investigator global assessment of response to therapy (Likert scale), 25 and 12.5 mg of rofecoxib compared with diclofenac (P = .99 and P = .01, respectively) were not identical, but were comparable with each other (P = .36) (Table 3). Significant differences resulted from sample sizes, which needed to be large to ensure satisfaction of the comparability criteria. In secondary analyses of last observed values, and an analysis considering data from only those patients who completed a full year of treatment, findings were consistent with the primary modified intention-to-treat approach for all end points, which confirms the robustness of these data.
Similar rates of acetaminophen use for rescue were seen between 25 mg of rofecoxib and diclofenac, but not between 12.5 mg of rofecoxib and diclofenac (P = .13 and P = .01, respectively) and between doses of rofecoxib (P = .30) and for all groups for discontinuation due to lack of efficacy (P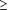 .16) (Table 2). Study joint tenderness was significantly improved from baseline, and reported at similar magnitudes among patients receiving rofecoxib at 12.5 and 25 mg or diclofenac (P .16) (Table 2). Study joint tenderness was significantly improved from baseline, and reported at similar magnitudes among patients receiving rofecoxib at 12.5 and 25 mg or diclofenac (P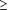 .66) (Table 3). .66) (Table 3).
Treatment effects were seen at the first measurement of efficacy and sustained throughout the 52-week treatment period (Figure 4). No medication for OA other than study therapy was permitted during the first 26 weeks of the trial, and few patients (<7%) in any treatment group used concomitant OA therapies in the second 26 weeks of the study (Table 2).
SAFETY
Deaths
No deaths occurred in the 6-week ibuprofen study. In the 1-year diclofenac study, 4 deaths occurred in the diclofenac group; none occurred in the rofecoxib groups (P<.05 for diclofenac vs rofecoxib). No death was considered to be drug-related by the investigator, and 1 patient had discontinued study medication 11 days before the event causing death. The causes of death were myocardial infarction, suicide, cerebrovascular accident, and postoperative complication. Two of these patients were older than 75 years, and all had previous medical history of related disorders, including previous myocardial infarctions, depression, and hypertension.
Discontinuations Due to Adverse Experiences
In the 6-week ibuprofen study, 39 patients discontinued from the study due to adverse experiences. No significant between-group differences were observed among patients who discontinued therapy. Twenty-two patients discontinued for GI adverse experiences (most commonly abdominal pain, dyspepsia, and epigastric discomfort): 2 who received placebo, 5 receiving 12.5-mg rofecoxib, 8 receiving 25-mg rofecoxib, and 7 receiving ibuprofen. Four patients discontinued due to cardiovascular adverse experiences (cerebrovascular accident and coronary artery disease, atrial arrhythmia, and unstable angina): 1 receiving placebo, 1 receiving 12.5-mg rofecoxib, and 2 receiving 25-mg rofecoxib. Other reasons for discontinuation were varied (headache, dizziness, edema, urticaria, oral ulcer, bursitis, somnolence, and fatigue), and did not occur more frequently in any 1 treatment group.
In the 1-year diclofenac study, 86 patients discontinued because of adverse experiences. Significantly more patients in the diclofenac group discontinued because of adverse experiences compared with those receiving rofecoxib (P<.05 vs 12.5 and 25 mg of rofecoxib, respectively). Forty patients discontinued due to adverse GI experiences, most commonly epigastric discomfort, diarrhea, dyspepsia, and nausea. Nine of these patients were receiving 12.5 mg of rofecoxib, 12 received 25 mg of rofecoxib, and 19 received diclofenac. Ten patients discontinued because of cardiovascular adverse experiences, including hypertension, palpitation, and transient ischemic attack: 3 who received 12.5 mg of rofecoxib, 4 receiving 25 mg of rofecoxib, and 3 receiving diclofenac. Eleven patients discontinued for elevated transaminase levels; 10 received diclofenac and 1 received rofecoxib at 25 mg (P<.05). Other reasons for discontinuation varied (rash, pyelonephritis, femoral fracture, anxiety, asthma, headache, edema, blurred vision, rales, hepatitis B, trauma, suicide attempt, osteonecrosis, tinnitus, back pain, depression, paresthesia, and epicondylitis) and did not occur significantly more frequently in any one treatment group.
1-YEAR SAFETY
Table 4 shows the adverse experiences occurring in 5% or more of patients in any individual treatment group, considered drug-related by investigators, over 1 year with rates of discontinuation. The rates of individual adverse experiences were generally similar between groups for all experiences listed, except abdominal pain, which occurred at a significantly higher rate in the diclofenac group compared with rofecoxib (P = .01 vs 12.5 and 25 mg of rofecoxib, respectively).
|
|

|
Table 4. Patients With Drug-Related Clinical Adverse Experiences (Incidence 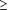 5%)* 5%)*
|
|
|
COMMENT
Rofecoxib, a COX-2 inhibitor, is a new treatment for OA, a common chronic disease requiring long-term therapy. The data presented above demonstrate that rofecoxib is effective in treating the signs and symptoms of OA, with once-daily administration. Rofecoxib's efficacy was superior to placebo and comparable with nonselective NSAIDs (ibuprofen and diclofenac) commonly used in treating this disease. The data showed similar efficacy in 2 studies of patients with OA of the knee and hip, over 1 year of treatment. Results were evident at the first assessment after treatment and sustained over the treatment period in each study. Most patients responded to treatment with rofecoxib, as assessed by the patient global assessment of response to therapy.
In the 1-year diclofenac study, a majority of patents completed a full year of therapy. Patients who discontinued from the trial early and those who completed a full year of treatment showed generally similar rates of response to therapy, as assessed by analyses of all observed patient data and data from patients who completed a full year of treatment. Only a small number of patients opted to use supplemental medication for OA pain in the second 26 weeks of the study, indicating that most patients had sufficient relief from pain, disability, and stiffness with the active agents in the study.
According to strict criteria, rofecoxib showed comparable efficacy with anti-inflammatory doses of NSAIDs currently used to treat OA, 2400 mg of ibuprofen and 150 mg of diclofenac, from the first measurement of efficacy through up to 1 year of treatment. This comparability was measured using guidelines established by OMERACT, an international consensus group, designed to show clinically perceptible differences in treatment effects. Small, but significant, differences between 12.5 mg of rofecoxib and diclofenac occurred within these prespecified comparability bounds. These findings may have resulted from the large sample sizes required to obtain sufficient power to yield 95% CIs within the prespecified comparability bounds.
In the studies reported above, a single morning dose of rofecoxib has similar efficacy in treating OA when compared with NSAIDs, which require 3 daily doses. Individual questions about night pain and morning stiffness from the WOMAC subscales provided results consistent with a treatment effect that was sustained over the 24-hour dosing interval. Published data on dental pain also demonstrate sustained analgesic efficacy with rofecoxib over 24 hours.27 Previously published pharmacokinetic data indicated that rofecoxib would provide a long duration of efficacy from a single dose.28-31
Rofecoxib was generally safe and well tolerated in the studies reported above. No deaths occurred in any patient taking rofecoxib in either study. Few patients discontinued therapy because of adverse experiences; rates were generally similar between the active treatment groups. In the 1-year diclofenac study, rofecoxib revealed no unanticipated safety issues. Dyspeptic symptoms were the most common adverse experiences causing patient discontinuation, in all treatment groups, including placebo. Overall, discontinuation rates because of specific GI dyspeptic symptoms were low in both studies, indicating that the drug (and its comparators) was well tolerated in the individual study populations.
The rationale for developing COX-2 inhibitors is the potential for improved GI safety over widely used nonspecific NSAIDs. The sample sizes of the 2 studies currently reported were insufficient to compare the relative safety of rofecoxib and the nonselective NSAID comparators. However, in a combined analysis of 8 phase 2b/3 OA trials, including the 2 trials reported here, there was a lower overall incidence of significant gastromucosal injuries, specifically perforations, ulcerations, or bleeding over 1 year in patients taking rofecoxib, compared with patients taking either diclofenac or ibuprofen.32
Renal safety is an especial concern in any chronic condition requiring long-term therapy with agents that inhibit COX. However, since COX-2 and COX-1 are both constitutively expressed in the kidney,33 COX-2 inhibitors are expected to have a similar renal safety profile to nonselective NSAIDs. That is, COX-2 inhibition is not expected to be less renally toxic than COX-1/COX-2 inhibition. Overall, approximately 5% of patients taking NSAIDs will have clinically detectable fluid retention, the most commonly reported renal effect among patients taking NSAIDs.34 In the studies reported above, the overall rates of edema and fluid retention were low, and drug-related events did not occur at a rate of more than 5% in any treatment group, including NSAID comparators, in either study. Sample sizes were too small to make claims about specific renal adverse effects, and further examination of larger databases will be required to develop this information.
We reported data from 2 separate trials to emphasize the effectiveness of rofecoxib, in 2 different patient populations. In the 2 trials (1 conducted in the United States, and 1 conducted multinationally), rofecoxib showed a similar degree of efficacy. However, further study is required to identify any specific patient populations with decreased or increased sensitivity or response to rofecoxib.
In the 2 studies shown above, a 6-week ibuprofen study and a 1-year diclofenac study, rofecoxib, a COX-2 inhibitor, was generally safe and well tolerated and produced clinically important improvements in the symptoms of OA over 1 year, at all measured intervals. Similar treatment responses were seen with both 12.5 and 25 mg of rofecoxib, and at 6 weeks and 1 year. These responses were consistent in the 2 studies shown, in distinct groups of patients, both in the United States and internationally. Furthermore, rofecoxib administered once daily showed comparable efficacy with maximum-strength, multidose regimens of both ibuprofen and diclofenac. Durable efficacy over 6 and 52 weeks make rofecoxib an important addition to the therapeutic armamentarium for osteoarthritis.
AUTHOR INFORMATION
Accepted for publication July 25, 2000.
Supported by a grant from Merck & Co Inc, Rahway, NJ.
We thank Michael Lee, Saurabh Mukhopadyay, MD, David Krupa, Amy Ko, and Frank Cihon for providing statistical analysis; Paige Reagan, Lori Geissler-Thorn, Magaly Garcia-Woolard, Merle Schneider, John Benedetto, Yvette Ng, and Mary Carson for performing vital internal monitoring; Michael Chou for assembling additional data tables; Tracey Malloy for facilitating the article planning process; Elliot Ehrich, MD, and Beth C. Seidenberg, MD, for vital imput into the study design and early drafts of this article; and Elliot Ehrich, MD, for designing both of the studies reported in this trial. Dr Samara thanks the following team members: Manoel B. Bertolo, Ibsen B. Coimbra, Lilian T. L. Constellat, and Sandra R. M. Fernandes, for their contributions.
Investigators for the 6-Week Ibuoprofen and 1-Year Diclofenac Studies
The following investigators enrolled patients and conducted study procedures:
6-Week Ibuprofen Study
Alabama: Barry McLean, Birmingham; Melvin Russell, Tallahassee; William Shergy, Huntsville; John Upchurch, Birmingham.
Arizona: Gerald Shockey, Mesa; John R. P. Tesser, Phoenix.
California: Daniel Arkfeld, Los Angeles; James E. Lewis, Sunnyvale; Lawrence P. McAdam, Thousand Oaks; C. Michael Neuwelt, San Leandro; Robin K. Dore, Anaheim; Margaret Drehobl, San Diego.
Colorado: Eugene Miller, Colorado Springs; William W. Storms, Colorado Springs.
Connecticut: Paul Dalgin, Stamford.
Delaware: James H. Newman, Wilmington.
Florida: Russell Graham, Altamonte Springs; Harris McIlwain, Tampa; Alastair Kennedy, Melbourne; Fernando Larach, St Petersburg; Michael C. Schweitz, West Palm Beach.
Georgia: Daniel J. Appelrouth, Atlanta.
Kentucky: Paul Goldfarb, Lexington; David Neustadt, Louisville; Michael Lee Peveler, Louisville.
Maryland: Herbert Baraf, Wheaton; Nathan Wei, Frederick.
Massachusetts: William Lloyd, Salem; S. David Miller, North Dartmouth; Robert Yood, Worcester.
New Jersey: Marc Goldberg, Passaic; Sheldon Solomon, Cherry Hill.
New Mexico: Steven Hsi, Albuquerque; Lance Rudolph, Albuquerque.
New York: Barry Gruber, East Setauket; Alan T. Kaell, Smithtown.
North Carolina: Gregory Collins, Charlotte; Thomas Littlejohn III, Winston-Salem; Ahmad Kashif, Charlotte; John Rubino, Raleigh.
Ohio: Beverly Carpenter, Cincinnati; Michael Luggen,Cincinnati; David Mandell, Mayfield Village.
Oklahoma: James McKay, Tulsa.
Oregon: Peter Bonafede, Portland; Jerome Brem, Portland; W. Michael Ryan, Portland.
Pennsylvania: Charles Ludivico, Bethlehem; Michael Franklin, Willow Grove; Alan J. Kivitz, Altoona.
Tennessee: Jon H. Levine, Nashville; Michael A. McAdoo, Milan.
Texas: Dennis Ruff, San Antonio.
Vermont: Richard P. Tonino, Burlington.
Washington: Robert Bettis, Edmonds; John Richard Newton, Wenatchee; Steve Overman, Seattle; Stephen R. Shaul, Yakima.
1-Year Diclofenac Study
Argentina: Alberto Romanowicz, Buenos Aires.
Belgium: Piet Geussens, Diepenbeek; J Reginster, Liege; J. Devogelare, Bruxelles.
Brazil: Joao Brenol, Porto Alegre; Adil Samara, Sao Paulo.
Canada: Catherine Alderdice, St John's; Nicholas Bellamy, London; William Bensen, Hamilton; Simon Huang, Vancouver; Gunnar Kraaj, Ottowa; Robert Inman, Toronto.
Costa Rica: Hernan Garcia, San Jose.
Denmark: Claus Hellesen, Glostrup; Lisbeth Krohn, Copenhagen.
England: R. Bernstein, Manchester; C. Black, London; B. Hazelman, Cambridge; Michael Webley, Aylesbury.
Finland: Esko Alhava, Kupio; Martti Hamalainen, Oulo.
France: Maxime Dougados, Paris; Xavier Le Loet, Rouen; Philippe Tauveron, Tours.
Germany: Dirk Ganzer, Griefswald; Frank Mielke, Berlin; Bernhard Weigl, Rosenheim.
Guatemala: Eduardo Samayoa, Guatemala City.
Hungary: Gyula Poor, Budapest.
Iceland: Helgi Jonsson, Reykjavik.
Italy: I. Caruso, Milan.
Mexico: Ruben Burgos, Mexico City.
Peru: Eduard Acevedo, Lima.
Russia: V. Nasonova, Moscow.
South Africa: Stan Brighton, Pretoria; Asgar Kalla, Cape Town.
Spain: Alberto Alonso, Baracaldo; Raimon San Marti, Barcelona; Vicente Valverde, Santander.
Sweden: Raynald Katziola, Frolunda; Jan Lidstrom, Kunsbaka.
Corresponding author: Lisa DeTora, PhD, Merck Research Laboratories, RY32-629, Rahway, NJ 07065 (e-mail: lisa_detora{at}merck.com).
From the Division of Clinical Immunology and Rheumatology, University of Alabama, Birmingham (Dr Saag); the Department of Rheumatology, Maastricht University Hospital, Maastricht, the Netherlands (Dr van der Heijde); the Tidewater Physicians Multispecialty Group, Newport News, Va (Dr Fisher); the Department of Rheumatology, Hospital das Clinicas-UNICAMP, Campinas, Sao Paulo, Brazil (Dr Samara); and the Merck Research Laboratories, Merck & Co, Rahway, NJ (Drs DeTora, Sperling, and Daniels and Mr Bolognese). Drs Saag, van der Heijde, and Samara have received honoraria and served as consultants to Merck & Co Inc.
REFERENCES
 |  |
1. Not Avavilable. Prevalence and impact of arthritis among women–United States, 1989-1991. MMWR Morb Mortal Wkly Rep. 1995;44:329-334.
PUBMED
2. Cooper C. Osteoarthritis and related disorders: epidemiology. In: Klippel JH, Dieppe PA, Arnett FC, et al, eds. Rheumatology. 2nd ed. St Louis, Mo: Mosby–Year Book Inc; 1998.
3. Mazzuca SA, Brandt KD, Katz BP, Li W, Stewart KD. Therapeutic strategies distinguish community based primary care physicians from rheumatologists in the management of osteoarthritis. J Rheumatol. 1993;20:80-86.
ISI
| PUBMED
4. Bullough PG. Osteoarthritis and related disorders: pathology. In: Klippel JH, Dieppe PA, Arnett FC, et al, eds. Rheumatology. 2nd ed. St Louis, Mo: Mosby–Year Book Inc; 1998.
5. Dieppe P, Lim K. Osteoarthritis and related disorders: clinical features and diagnostic problems. In: Klippel JH, Dieppe PA, Arnett FC, et al, eds. Rheumatology. 2nd ed. St Louis, Mo: Mosby–Year Book Inc; 1998.
6. Creamer P, Hochberg MC. Osteoarthritis. Lancet. 1997;350:503-508.
FULL TEXT
|
ISI
| PUBMED
7. Zhang Y, Hannan MT, Chaisson CE, et al. Bone mineral density and risk of incident and progressive radiographic knee osteoarthritis in women: the Framingham Study. J Rheumatol. 2000;27:1032-1037.
ISI
| PUBMED
8. American Academy of Orthopedic Surgeons. Research Committee Fact Sheet: osteoarthritis. Available at: http://www.aaos.org/wordhtml/research/oa.htm. Accessibility verified August 24, 2000.
9. Altman R, Asch D, Bloch G, et al for the Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Development of criteria for the classification and reporting of osteoarthritis: classification of osteoarthritis of the knee. Arthritis Rheum. 1986;29:1039-1049.
ISI
| PUBMED
10. Bellamy N. Osteoarthritis. In: Bellamy N, ed. Musculoskeletal Clinical Metrology. Norwell, Mass: Kluwer Academic Publishers; 1993:169-176.
11. Brandt KD. Management of osteoarthritis. In: Kelley WN, Ruddy S, Harris EDJ, Sledge CB, eds. Textbook of Rheumatology. 5th ed. Philadelphia, Pa: WB Saunders Co; 1997:1394-1403.
12. Petersson IF. Osteoarthritis and related disorders: the assessment of process and outcome in osteoarthritis. In: Klippel JH, Dieppe PA, Arnett FC, et al, eds. Rheumatology. 2nd ed. St Louis, Mo: Mosby–Year Book Inc; 1998.
13. Peyron JG. The epidemiology of osteoarthritis. In: Moskowitz RW, Howell DS, Goldberg VM, Mankin HJ, eds. Osteoarthritis: Diagnosis and Management. Philadelphia, Pa: WB Saunders Co; 1984:9-27.
14. Dieppe P, Buckwalter JA. Osteoarthritis and related disorders: management of limb joint osteoarthritis. In: Klippel JH, Dieppe PA, Arnett FC, et al, eds. Rheumatology. 2nd ed. St Louis, Mo: Mosby–Year Book Inc; 1998.
15. Hochberg MC, Altman RD, Brandt KD, et al for the American College of Rheumatology. Guidelines for the medical management of osteoarthritis, part I: osteoarthritis of the hip. Arthritis Rheum. 1995;38:1535-1540.
ISI
| PUBMED
16. Hochberg MC, Altman RD, Brandt KD, et al for the American College of Rheumatology. Guidelines for the medical management of osteoarthritis, part II: osteoarthritis of the knee. Arthritis Rheum. 1995;38:1541-1546.
ISI
| PUBMED
17. Baum C, Kennedy DL, Forbes MB. Utilization of nonsteroidal anti-inflammatory drugs. Arthritis Rheum. 1985;28:686-692.
ISI
| PUBMED
18. Fries JF. NSAID gastropathy: the second most deadly rheumatic disease? epidemiology and risk appraisal. J Rheumatol. 1991;18:6-10.
19. Fries JF, Williams CA, Bloch DA, Michel BA. Nonsteroidal anti-inflammatory drug-associated gastropathy: incidence and risk factor models. Am J Med. 1991;91:213-222.
FULL TEXT
|
ISI
| PUBMED
20. Wolfe MM, Lichtenstein DR, Singh G. Gastrointestinal toxicity of nonsteroidal antiinflammatory drugs. N Engl J Med. 1999;340:1888-1899.
FREE FULL TEXT
21. Battistini B, Botting R, Bakhle YS. COX-1 and COX-2: toward the development of more selective NSAIDs. DN&P. 1994;7:501-512.
22. Lanza FL. Gastrointestinal toxicity of newer NSAIDs. Am J Gastroenterol. 1993;88:1318-1323.
ISI
| PUBMED
23. Bellamy N, Carette S, Ford PM, et al. Osteoarthritis antirheumatic drug trials, III: setting the delta for clinical trials—results of a consensus development (Delphi) exercise. J Rheumatol. 1992;19:451-457.
ISI
| PUBMED
24. Bellamy N, Kirwan J, Boers M, et al. Recommendations for a core set of outcome measures for future phase III clinical trials in knee, hip, and hand osteoarthritis: consensus development at OMERACT III. J Rheumatol. 1997;24:799-802.
ISI
| PUBMED
25. Hochberg MC, Chang RW, Dwosh I, Lindsey S, Pincus T, Wolfe F. The American College of Rheumatology 1991 revised criteria for the classification of global functional status in rheumatoid arthritis. Arthritis Rheum. 1992;35:498-502.
ISI
| PUBMED
26. Hochberg MC, Altman RD, Brandt KD, Moskowitz RW. Design and conduct of clinical trials in patients with osteoarthritis: preliminary recommendations from a task force of the Osteoarthritis Research Society. J Rheumatol. 1997;24:792-794.
PUBMED
27. Sigthorsson G, Crane R, Simon T, et al. Cox-2 specific inhibition with rofecoxib does not increase intestinal permeability in healthy subjects: a double-blind, crossover study comparing rofecoxib with placebo and indomethacin. Gut. 2000;47:527-532.
FREE FULL TEXT
28. Schwartz JI, VanHecken A, DeLepeleire I, et al. Comparative activity of rofecoxib (MK-0966, VIOXX), meloxicam, diclofenac, buprofen and naproxen on COX-2 vs COX-1 in healthy female volunteers. Presented at: the 14th Congress of the European League Against Rheumatism; June 6-11, 2000; Glasgow, Scotland.
29. Chan CC, Prasit P, Riendeau D. Preclinical biological profile of rofecoxib (vioxx,mk-0966): a potent, orally active and highly selective cox-2 inhibitor [abstract]. Mediators Inflamm. 1999;8:S132-S132.
30. Chan CC, Boyce S, Brideau C, et al. Rofecoxib [Vioxx, MK-0966; 4-(4'-methylsulfonylphenyl)-3-phenyl-2-(5H)-furanone]: a potent and orally active cyclooxygenase-2 inhibitor: pharmacological and biochemical profiles. J Pharmacol Exp Ther. 1999;290:551-560.
FREE FULL TEXT
31. Prasit P, Wang Z, Brideau C, et al. The discovery of rofecoxib, [MK 966, Vioxx,4-(4'-methylsulfonylphenyl)-3-phenyl-2(5H)-furanone], an orally active cyclooxygenase-2-inhibitor. Bioorg Med Chem Lett. 1999;9:1773-1778.
FULL TEXT
| PUBMED
32. Langman MJ, Jensen DM, Watson DJ, et al. Adverse upper gastrointestinal effects of rofecoxib compared with NSAIDs. JAMA. 1999;282:1929-1933.
FREE FULL TEXT
33. Crofford LJ. COX-1 and COX-2 tissue expression: implications and predictions. J Rheumatol. 1997;24:15-19.
34. Whelton A, Hamilton CW. Nonsteroidal anti-inflammatory drugs: effects on kidney function. J Clin Pharmacol. 1991;31:588-598.
ABSTRACT
THIS ARTICLE HAS BEEN CITED BY OTHER ARTICLES
 |
Guest Authorship and Ghostwriting in Publications Related to Rofecoxib: A Case Study of Industry Documents From Rofecoxib Litigation
Ross et al.
JAMA 2008;299:1800-1812.
ABSTRACT
| FULL TEXT
Non-steroidal anti-inflammatory drugs and myocardial infarctions: comparative systematic review of evidence from observational studies and randomised controlled trials
Scott et al.
Ann Rheum Dis 2007;66:1296-1304.
ABSTRACT
| FULL TEXT
Efficacy and safety of etoricoxib 30 mg and celecoxib 200 mg in the treatment of osteoarthritis in two identically designed, randomized, placebo-controlled, non-inferiority studies
Bingham et al.
Rheumatology (Oxford) 2007;46:496-507.
ABSTRACT
| FULL TEXT
Adverse Effects of Cyclooxygenase 2 Inhibitors on Renal and Arrhythmia Events: Meta-analysis of Randomized Trials
Zhang et al.
JAMA 2006;296:1619-1632.
ABSTRACT
| FULL TEXT
Blood-nourishing and Hard-softening Capsule Costs Less in the Management of Osteoarthritic Knee Pain: A Randomized Controlled Trial
Cao et al.
Evid Based Complement Alternat Med 2005;2:363-368.
ABSTRACT
| FULL TEXT
Evaluation of the Comparative Efficacy of Etoricoxib and Ibuprofen for Treatment of Patients With Osteoarthritis: A Randomized, Double-Blind, Placebo-Controlled Trial
Wiesenhutter et al.
Mayo Clin Proc. 2005;80:470-479.
ABSTRACT
Medicaid Prior-Authorization Programs and the Use of Cyclooxygenase-2 Inhibitors
Fischer et al.
NEJM 2004;351:2187-2194.
ABSTRACT
| FULL TEXT
Cyclooxygenase Isozymes: The Biology of Prostaglandin Synthesis and Inhibition
Simmons et al.
Pharmacol. Rev. 2004;56:387-437.
ABSTRACT
| FULL TEXT
Effects of ibuprofen on molecular markers of cartilage and synovium turnover in patients with knee osteoarthritis
Gineyts et al.
Ann Rheum Dis 2004;63:857-861.
ABSTRACT
| FULL TEXT
Gastrointestinal Tolerability and Effectiveness of Rofecoxib versus Naproxen in the Treatment of Osteoarthritis: A Randomized, Controlled Trial
Lisse et al.
ANN INTERN MED 2003;139:539-546.
ABSTRACT
| FULL TEXT
Incidence of gastroduodenal ulcers in patients with rheumatoid arthritis after 12 weeks of rofecoxib, naproxen, or placebo: a multicentre, randomised, double blind study
Hawkey et al.
Gut 2003;52:820-826.
ABSTRACT
| FULL TEXT
The Cost-Effectiveness of Cyclooxygenase-2 Selective Inhibitors in the Management of Chronic Arthritis
Spiegel et al.
ANN INTERN MED 2003;138:795-806.
ABSTRACT
| FULL TEXT
Results of a randomized, dose-ranging trial of etoricoxib in patients with osteoarthritis
Gottesdiener et al.
Rheumatology (Oxford) 2002;41:1052-1061.
ABSTRACT
| FULL TEXT
|