 |
 |

Antiviral Therapy for Herpes Zoster
Randomized, Controlled Clinical Trial of Valacyclovir and Famciclovir Therapy in Immunocompetent Patients 50 Years and Older
Stephen K. Tyring, MD, PhD;
Karl R. Beutner, MD, PhD;
Bruce A. Tucker, MD;
Walter C. Anderson, MD;
R. Jane Crooks, PhD
Arch Fam Med. 2000;9:863-869.
ABSTRACT
 |  |
Objective To compare the efficacy and safety of valacyclovir hydrochloride and famciclovir for the treatment of herpes zoster.
Design A double-blind, randomized, controlled, multicenter clinical trial in which patients received 7 days of treatment and were followed up for 24 weeks.
Settings Patients reported directly to specialist centers or were referred from primary care centers.
Patients There were 597 otherwise healthy immunocompetent outpatients, aged 50 years and older, who presented within 72 hours of onset of zoster rash.
Interventions Treatment with valacyclovir hydrochloride (1 g 3 times daily) or famciclovir (500 mg 3 times daily) for 7 days.
Main Outcome Measures Resolution of zoster-associated pain and postherpetic neuralgia, rash healing, and treatment safety.
Results Intent-to-treat analysis did not detect statistically significant differences for valacyclovir vs famciclovir on resolution of zoster-associated pain (hazard ratio, 1.02; 95% confidence interval, 0.84-1.23; P = .84). Furthermore, no differences were evident between treatments on rash healing rates and on a range of analyses of postherpetic neuralgia. Safety profiles for valacyclovir and famciclovir were similar, with headache and nausea being the more common adverse events.
Conclusions Valacyclovir treatment is comparable to famciclovir treatment in speeding the resolution of zoster-associated pain and postherpetic neuralgia. Current wholesale prices indicate that valacyclovir is the more cost-effective treatment for herpes zoster ($83.90 vs $140.70 per course).
INTRODUCTION
ACUTE HERPES zoster is a painful, debilitating condition, especially in older adults. The pain of herpes zoster is the principal reason most patients seek medical attention from their primary care physician.1 As age increases, the risk of zoster-associated pain persisting after rash healing also increases.2-3 The resultant chronic pain, often referred to as postherpetic neuralgia, is difficult and often costly to treat effectively.4-5 Antiviral therapy for the treatment of herpes zoster, therefore, must alleviate the early symptoms and favorably affect outcome on chronic pain and postherpetic neuralgia.
In recent years, valacyclovir hydrochloride (Valtrex; Burroughs Wellcome Co, Research Triangle Park, NC) and famciclovir (Famvir; SmithKline Beecham Pharmaceuticals, Philadelphia, Pa) have been developed with the aim of improving on oral acyclovir, a widely recognized standard of care for the treatment of herpes zoster.6-7 Valacyclovir and famciclovir are the oral prodrugs of acyclovir and penciclovir, respectively. Although the active moieties are categorized as nucleoside analogs, their intracellular kinetics and target site mode of action in inhibiting replication of varicella zoster virus DNA are different.8-9 It is therefore important that their efficacy and safety profiles in clinical use are compared objectively so that physicians can make a well-informed choice about which drug to prescribe. Valacyclovir and famciclovir are given 3 times daily for 7 days, which should enhance compliance compared with acyclovir, which is given 5 times daily. The approved valacyclovir hydrochloride dosage worldwide is 1 g 3 times daily. In the United States, famciclovir, 500 mg 3 times daily, is the approved regimen, but in most other countries the dosage is 250 mg 3 times daily.
Publications of controlled, randomized clinical trials are the mainstay of evidence to support claims for efficacy and to provide salient information on safety. Once efficacy and safety have been established, other aspects, eg, costs and cost-effectiveness analyses, may be introduced into decision making and development of treatment guidelines. Such studies6-7,10-11 have now been published for valacyclovir and famciclovir, along with additional reports of economic analyses, although so far these have been based only on comparison with acyclovir or placebo. The superiority of valacyclovir therapy in speeding resolution of zoster-associated pain compared with acyclovir therapy has been identified, along with additional analyses of its advantages on postherpetic neuralgia.6 A benefit of famciclovir treatment (500 mg) in resolving postherpetic neuralgia has been demonstrated vs placebo, but the study7 used different analysis methods and was not reported in association with an overall analysis of zoster-associated pain. A second study12 of famciclovir treatment described its performance on zoster-associated pain relative to acyclovir treatment but does not report its impact on postherpetic neuralgia. The prescribing physician consequently has to judge the relative merits of each drug, then relate these to the needs and risks for an individual patient, often in the absence of complete or truly comparable data. Factors that can confound a balanced assessment of available information include differences in trial design, patient selection criteria, primary clinical end points, and data analysis methods.
This randomized, double-blind, controlled clinical trial directly comparing valacyclovir and famciclovir as treatments for acute herpes zoster was conducted to address some of the limitations of available published data on these agents. The emphasis was placed on zoster-associated pain and postherpetic neuralgia, evaluating the most relevant age group of 50 years and older, a population for whom antiviral therapy is usually recommended and in whom the risk of developing these sequelae is substantial.13-15
PATIENTS AND METHODS
STUDY DESIGN
This was a double-blind, randomized, parallel, multicenter comparison of the efficacy and safety of valacyclovir hydrochloride, 1 g 3 times daily, and famciclovir, 500 mg 3 times daily, as treatments for acute herpes zoster. Patients received study medication for 7 days and were followed up for 24 weeks.
PATIENTS
Patients were referred from primary care centers or presented directly to specialist referral centers in the United States. Eligible patients were otherwise healthy, immunocompetent adults aged 50 years and older with clinically diagnosed (ie, signs and symptoms consistent with the diagnosis) localized herpes zoster presenting within 72 hours of the onset of rash. Patients with herpes zoster ophthalmicus (defined as cutaneous lesions in the dermatome associated with the ophthalmic division of the trigeminal nerve) were excluded because prescribing information for famciclovir (United States) indicated a lack of clinical experience in such patients at the time this study commenced.16 Pregnant, nursing, and sexually active women of childbearing potential were excluded, as were patients who had received cytotoxic or immunosuppressive drug therapy within the 3 months before presentation. Patients who had received topical or systemic antiviral medications or immunomodulatory agents for varicella zoster virus infections, eg, interferon or capsaicin, within the previous 4 weeks and those receiving tricyclic antidepressant drugs or probenecid immediately before presentation were not eligible. Also excluded were patients with congenital, acquired, or corticosteroid-induced immunodeficiency, including malignancy, significantly impaired renal function (estimated creatinine clearance of  0.50 mL/s [ 0.50 mL/s [ 30 mL/min]), impaired hepatic function (alanine or aspartate aminotransferase levels >5 times the upper limit of the reference range), or a history of intolerance or hypersensitivity to acyclovir, penciclovir, valacyclovir, or famciclovir. 30 mL/min]), impaired hepatic function (alanine or aspartate aminotransferase levels >5 times the upper limit of the reference range), or a history of intolerance or hypersensitivity to acyclovir, penciclovir, valacyclovir, or famciclovir.
Institutional review board approval was obtained at each study site before enrollment commenced. Witnessed written informed consent was obtained from each patient before study participation.
STUDY PROCEDURES
Eligible patients were randomized (1:1) according to a computer-generated code to receive 7 days' treatment with valacyclovir hydrochloride, 1 g 3 times daily, or famciclovir, 500 mg 3 times daily. To preserve the double-blind nature of the trial, patients allocated to the valacyclovir group also received placebo famciclovir tablets and those allocated to the famciclovir group also received placebo valacyclovir tablets 3 times daily.
For patients randomized to the (active) famciclovir group, dosage adjustments were required for those with renal impairment, as defined by an estimated creatinine clearance of 0.50 to 0.98 mL/s (30-59 mL/min), to comply with approved dosage recommendations.16 In patients with a creatinine clearance of 0.67 to 0.98 mL/s (40-59 mL/min), the famciclovir dosage was reduced to 500 mg twice daily. In those with a creatinine clearance of 0.50 to 0.65 mL/s (30-39 mL/min), the famciclovir dosage was reduced to 500 mg once daily. No adjustments were considered necessary for the valacyclovir active dosage based on available pharmacokinetic information at the time of study initiation; placebo tablet administration (matching famciclovir) in valacyclovir recipients was adjusted according to creatinine clearance.
At enrollment (day 1) and before dosing, patients underwent a brief physical examination, and blood samples were obtained for hematologic (hemoglobin concentration, platelet count, and white blood cell count) and clinical chemistry (creatinine, alkaline phosphatase, and alanine or aspartate aminotransferase concentrations) analyses. The diagnosis of herpes zoster was clinically confirmed, and the date and time of onset of prodromal pain (if any) and rash were recorded. Pain intensity was recorded on a 6-point scale (none, just noticeable, mild, moderate, severe, and very severe). Rash severity was recorded as mild (<25 lesions), moderate (25-50 lesions), or severe (>50 lesions).
Patients were subsequently evaluated on days 3, 8, 14, and 28 and then every 4 weeks until week 24. Additional blood samples were obtained on days 3 and 8 for hematologic and clinical chemistry analyses. The rash was assessed until complete healing was evident, defined as 100% crusted or healed. Patients kept a diary daily (days 1-28) or weekly (weeks 4-24) in which they recorded information about their zoster-associated pain, burning, and other discomfort in the affected dermatome (according to the intensity categories described in the previous paragraph) and details about medications used to alleviate the pain. Patients were initially assessed in the clinic, but from day 28 forward assessments were at home or at clinic visits.
EFFICACY ASSESSMENT
The primary efficacy end point was time to complete cessation of zoster-associated pain, from which also was derived cessation of postherpetic neuralgia. Secondary end points included time to cessation of zoster-associated abnormal sensations, pain intensity, rash healing, and lesion dissemination. Additional measures of efficacy included in the original protocol were analgesic use, quality of life (Short Form 36 Health Status Survey), medical resource use, and the impact of pain on activity and sleep. However, results from these assessments are not presented in this article in view of the overall outcome on the primary and secondary clinical end points.
SAFETY ASSESSMENT
Safety was evaluated from hematologic and clinical chemistry monitoring and adverse events reported during days 1 to 10. Duration, intensity, severity, and the investigator's opinion on causality were recorded for each event. Adverse experiences considered serious were recorded throughout follow-up.
STATISTICAL METHODS
Sample Size
Assuming that, at most, 25% of patients treated with valacyclovir would still be experiencing pain at 6 months and that hazard functions are proportional, a sample size of 260 patients per treatment group provided 80% power to detect hazard ratios of 1.33 or higher and 0.75 or lower using the 2-tailed log-rank test at the 5% significance level.17
Analysis Methods
Demographic characteristics of the 2 treatment groups were compared using a 2-tailed Fisher exact test. The principal efficacy analysis was of the intent-to-treat population for zoster-associated pain. Further analyses of postherpetic neuralgia used intent-to-treat and subgroup methods previously described for valacyclovir and famciclovir.6-7 The intent-to-treat method to identify treatment differences on postherpetic neuralgia ascribed a duration of zero days for patients reporting no pain on or after rash healing or day 30, thus including all patients in the analysis.6 The subgroup method excluded patients with no pain on or after rash healing or day 30 from the analysis.7 An intent-to-treat analysis of time to loss of clinically significant zoster-associated pain was also performed. Pain was considered clinically significant if categorized as moderate or higher in intensity.
Distributions of each time-to-event end point (loss of pain, loss of abnormal sensations, loss of postherpetic neuralgia, and rash healing) were estimated by the Kaplan-Meier product limit survival method.18 Differences between treatments were determined using Cox proportional hazards models after adjusting for important prognostic factors known to affect outcome for that variable.19 For zoster-associated pain, postherpetic neuralgia, and abnormal sensations, these factors included age, presence of prodromal pain, pain severity at presentation, time between rash onset and start of treatment, and medical center.6-7,20 For secondary end points (proportions of patients with pain and with rash completely healed), point estimates and their associated 95% confidence intervals were derived. All significance tests were 2-sided.
Safety was assessed for all patients using visual inspection of adverse event reports and results of hematologic and clinical chemistry analyses.
RESULTS
DEMOGRAPHIC AND DISEASE CHARACTERISTICS
In total, 597 patients enrolled and were randomized to treatment with valacyclovir (n = 297) or famciclovir (n = 300). There were no major demographic differences between treatment groups, but some imbalances in disease characteristics at presentation were evident (Table 1). The median age was 68 years, and 73% of patients were older than 60 years. Overall, 63% of participants were women. More valacyclovir recipients reported that they had prodromal pain compared with those receiving famciclovir (78% vs 70%; P = .03). Median duration of prodromal pain was 2 to 3 days (57 and 69 hours for valacyclovir and famciclovir recipients, respectively). Prodromal pain was categorized as severe or very severe in intensity in 34% of valacyclovir recipients in contrast to 24% of famciclovir recipients (P = .03). More than 90% of the trial population had significant acute pain at presentation, with 71% and 69% of valacyclovir and famciclovir recipients, respectively, categorizing this as moderate or higher in intensity.
|
|

|
|
Table 1. Patient Demographic and Disease Characteristics at Presentation
|
|
|
Overall, 82% of patients commenced treatment within 48 hours of rash onset; 85% completed the study as protocolled. Reasons for premature discontinuation were evenly distributed between treatments. Key reasons were withdrawal of consent (6%); loss to follow-up (4%); protocol violation (3%); and adverse event, death, or inadequate response (<1% each).
EFFICACY
Intent-to-treat analysis did not detect a statistically significant difference between valacyclovir and famciclovir treatment on the resolution of zoster-associated pain (Figure 1 and Table 2). Prognostic factors identified as having an important effect on the duration of zoster-associated pain were advancing age (50-60 vs >60 years) and pain intensity at presentation ( mild vs mild vs 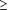 moderate pain). Because prodromal pain has been shown to affect pain outcome in other valacyclovir studies6, 20 and the time from rash onset to starting treatment was identified as important in famciclovir studies,12 these factors were also adjusted for in the Cox proportional hazards models for zoster-associated pain, postherpetic neuralgia, and abnormal sensations as presence vs absence and less than 48 vs 48 to 72 hours, respectively. The hazard ratio of 1.02 for valacyclovir vs famciclovir therapy for resolution of zoster-associated pain is close to unity, indicating a rate of pain resolution for valacyclovir not different from that for famciclovir. moderate pain). Because prodromal pain has been shown to affect pain outcome in other valacyclovir studies6, 20 and the time from rash onset to starting treatment was identified as important in famciclovir studies,12 these factors were also adjusted for in the Cox proportional hazards models for zoster-associated pain, postherpetic neuralgia, and abnormal sensations as presence vs absence and less than 48 vs 48 to 72 hours, respectively. The hazard ratio of 1.02 for valacyclovir vs famciclovir therapy for resolution of zoster-associated pain is close to unity, indicating a rate of pain resolution for valacyclovir not different from that for famciclovir.
|
|

|
|
Resolution of pain in patients with herpes zoster using valacyclovir hydrochloride (1 g 3 times daily) or famciclovir (500 mg 3 times daily).
|
|
|
|
|

|
|
Table 2. Valacyclovir Compared With Famciclovir Treatment for Resolution of Zoster-Associated Pain, Postherpetic Neuralgia (PHN), and Abnormal Sensations*
|
|
|
Proportions of patients with pain on or after rash healing were 86% and 87% for the valacyclovir and famciclovir groups, respectively. Proportions with pain at 1 month were 64% and 62% for the valacyclovir and famciclovir groups, respectively. At 3 months, 32% and 34% of valacyclovir and famciclovir recipients, respectively, still reported pain; at 6 months, 19% in each group still experienced pain. Table 2 lists the series of results for analysis of postherpetic neuralgia, detailing hazard ratios (and 95% confidence intervals) and an absence of statistical significance on any variable or method of analysis. Loss of clinically significant zoster-associated pain was similarly rapid with valacyclovir and famciclovir treatment (Table 2). Analysis for treatment by medical center (or group of smaller medical centers) interaction identified no specific medical center impact on treatment effects. As expected for the abnormal sensations end point, because it so closely allied pain and discomfort, no differences were detected between valacyclovir and famciclovir treatment (Table 2).
The impact of valacyclovir and famciclovir treatment on rash healing was virtually identical (hazard ratio, 1.01; 95% confidence interval, 0.93-1.30; P = .26). After 7 days, the rash was considered 100% crusted or healed in 32% of valacyclovir recipients and 25% of famciclovir recipients. By 14 and 28 days, the rash was observed to have healed in 89% and 96% of valacyclovir recipients and 82% and 99% of famciclovir recipients, respectively. No cases of cutaneous disseminated zoster were reported.
SAFETY
Thirty-four percent of valacyclovir recipients and 38% of famciclovir recipients reported 1 or more adverse events during the first 10 days of study. Most common were headache, nausea, and various gastrointestinal disturbances (Table 3). Most adverse events were mild in intensity and were not considered attributable to the use of study medications. Events were treatment limiting in 5 valacyclovir recipients (dizziness, loss of consciousness, gastrointestinal problems, folliculitis, and myocardial infarction) and 6 famciclovir recipients (gastrointestinal disturbances and insomnia). Adverse events were considered serious but unrelated to use of study drugs in 2 valacyclovir recipients (chest pain and myocardial infarction) and 4 famciclovir recipients (pneumonitis, pneumonia, cerebrovascular accident, "weakness," and exacerbation of urinary tract infection). Minor changes from baseline in hematologic and clinical chemistry variables were not considered clinically meaningful.
|
|

|
|
Table 3. Adverse Event Profiles for Patients Receiving Valacyclovir or Famciclovir*
|
|
|
COMMENT
This double-blind, randomized comparison of valacyclovir and high-dose famciclovir in acute herpes zoster did not detect differences between treatments on the main clinical outcome measures of zoster-associated pain, rash healing, and postherpetic neuralgia. The pattern of loss of pain appeared typical of the 3 phases—acute pain and early and late herpetic neuralgia—described previously.21 A limited series of additional analyses were consistent with the key findings. Furthermore, no differences in the adverse event profiles of the 2 drugs were evident.
Postherpetic neuralgia is the most feared complication of herpes zoster and, once established, is extremely difficult to manage effectively.4-5 Postherpetic neuralgia has a major impact on quality of life.5 Because it is more likely to occur in older patients, it is important to recognize that the impact of postherpetic neuralgia on quality of life could further add to the impairment associated with the range of other medical conditions typical of the age group.4, 6 The effect of famciclovir treatment on postherpetic neuralgia has previously been demonstrated for patients aged 50 years and older in a placebo-controlled trial.7 No similar study has been completed for valacyclovir treatment, and such a study would not be appropriate now given the more widespread acceptance that herpes zoster in individuals older than 50 years routinely merits antiviral therapy.15 However, the randomized, controlled study described herein provides confirmation that valacyclovir treatment too speeds resolution of postherpetic neuralgia to an extent comparable with that for famciclovir treatment.
Valacyclovir and famciclovir, as oral prodrugs of the acyclic nucleoside analogs acyclovir and penciclovir, respectively, depend on varicella zoster virus thymidine kinase for initial phosphorylation. Subsequent production of active acyclovir or penciclovir triphosphate is catalyzed by cellular enzymes. The intracellular kinetics of the triphosphates of acyclovir and penciclovir are different; the half-life of penciclovir triphosphate is notably longer than that of acyclovir triphosphate (9.0 vs 0.8 hours) in varicella zoster virus–infected cells.8 This may be an essential requirement of penciclovir triphosphate for termination of virus replication because it has a markedly lower inhibition constant compared with acyclovir triphosphate against varicella zoster virus DNA polymerase (1.60 vs 0.01 µmol/L).9 Our comparative study of valacyclovir and famciclovir in herpes zoster did not identify any therapeutic advantage of a prolonged intracellular triphosphate half-life.
The design and analysis of clinical trials of acute herpes zoster and prevention of postherpetic neuralgia has been a controversial topic in recent years.22-23 Measurement of zoster-associated pain preserves the key principle of intent-to-treat analysis by including all patients, but such statistical rigor tends to compromise the more clinically relevant challenge to detect treatment benefits on postherpetic neuralgia. Hence, various statistical methods have attempted to address this. Our study adopted analysis techniques previously applied to valacyclovir or famciclovir studies6-7 so that irrespective of methodological criticisms, like was compared with like. The results obtained show a highly consistent picture of the similarity of valacyclovir and famciclovir treatment on postherpetic neuralgia.
The performance of valacyclovir in this study, as indicated by median duration values for zoster-associated pain, was as expected from an earlier trial6 (42 vs 38 days). For famciclovir, the median duration of postherpetic neuralgia was shorter than in an earlier study7 (44 vs 63 days) when assessed using the same analysis method applied only to patients aged 50 years and older.
Some differences between treatment groups in disease and pain characteristics recorded at presentation were statistically significant, most notably prodromal symptoms. Our analyses of zoster-associated pain and postherpetic neuralgia accounted for the imbalance between treatment groups in presence vs absence of prodromal symptoms; however, it did not account for the statistically significant difference in the intensity of prodromal symptoms. More patients allocated to the valacyclovir group had more severe symptoms (11% more with prodromal symptoms and 42% more with severe prodromal pain). Because more intense or more prolonged prodromal pain and more severe acute pain have collectively been confirmed as important risk factors for prolonged chronic zoster-associated pain and postherpetic neuralgia,6, 20, 24-25 it is likely that these baseline disease characteristics masked detection of the possible superiority of valacyclovir treatment.
This study evaluated the famciclovir regimen approved in the United States (500 mg 3 times daily). In most other countries, the approved famciclovir regimen is 250 mg 3 times daily.26-27 No inference can be drawn from this or other currently published work regarding an impact of the 250-mg famciclovir regimen on postherpetic neuralgia.
Having established the comparability of valacyclovir and famciclovir (500 mg) in terms of clinical efficacy and safety in herpes zoster, the prescriber might want or need to evaluate treatment costs. In the absence of significant differences between the 2 treatments, only incremental costs unique to each therapy need to be considered from an economic perspective. In the United States, average wholesale costs for the recommended 7-day zoster treatment course are $83.90 and $140.70 for valacyclovir and famciclovir, respectively.28 Thus, valacyclovir is substantially more cost-effective (40%) than famciclovir for the treatment of herpes zoster and the prevention of painful sequelae.
This direct comparison addressed the several limitations that, to date, have precluded a balanced assessment of the relative merits and limitations of published information on valacyclovir and famciclovir as treatments for acute herpes zoster. The similarity demonstrated for valacyclovir and the 500-mg famciclovir regimen highlights the overall value of valacyclovir, particularly in relation to its therapeutic benefits on chronic zoster-associated pain or postherpetic neuralgia.
AUTHOR INFORMATION
Accepted for publication June 27, 2000.
This study was supported by a grant from Glaxo Wellcome Inc, Research Triangle Park, NC.
We are indebted to Mitch Miller for his major contribution to the conduct of the study and to Gosford Sawyerr, Ken Krall, and Greg Giguere for statistical analysis.
We also acknowledge all participating clinical investigators: W. Anderson, New Braunfels, Tex; F. Aoki, Winnipeg, Manitoba; S. Bean, Houston, Tex; K. Beutner, Vallejo, Calif; Daniel Bixler, Omaha, Neb; L. Davis, San Antonio, Tex; M. Brandon, San Diego, Calif; D. Buntin, Hermitage, Tenn; F. Bures, Winona, Minn; N. Cannon, Birmingham, Ala; R. Charles, Petosky, Mich; S. Clark, Longmont, Colo; L. Cleaver, Kirksville, Mo; F. Cole, Fayetteville, Ga; H. Collins, Edison, NJ; J. Duff, Springfield, Mo; J. Fairfield, Lansdale, Pa; J. Fidelholtz, Cincinnati, Ohio; C. Forszpaniak, Naples, Fla; B. Gainer, Morgantown, WVa; M. Goldman, Encinitas, Calif; J. Hanifin, Portland, Ore; M. Henry, Rhinelander, Wis; C. Hughes, Evansville, Ind; M. Huston-Kmiec, Aurora, Ill; J. Kalivas, Phoenix, Ariz; D. Krichbaum, South Bend, Ind; S. Kraus, Atlanta, Ga; T. Kurtz, Waukee, Iowa; T. Lechmaier, Madison, Wis; C. Lynde, Markham, Ontario; T. McKnight, Fremont, Neb; D. Mee-Lee, Honolulu, Hawaii; D. Mikolich, Providence, RI; E. Monroe, Milwaukee, Wis; T. Nigra, Washington, DC; A. Oppenheim, San Rafael, Calif; G. Pankey, New Orleans, La; K. Papp, Waterloo, Ontario; J. Portnoy, Montreal, Quebec; J. Powers, Scottsdale, Ariz; T. Rist, Knoxville, Tenn; J. Rivers, Vancouver, British Columbia; S. Sacks, Vancouver; S. Scheibner, Statesville, NC; C. Schupbach, Charlotte, NC; S. Shah, Houston; G. Silverman, Vero Beach, Fla; D. Silvers, Los Angeles, Calif; C. St. Pierre, Sherbrooke, Quebec; D. Stevens, Boise, Idaho; D. Stryker, Alameda, NM; J. Taylor, Miami, Fla; S. Trottier, Ste Foy, Quebec; E. Tschen, Albuquerque, NM; B. Tucker, Birmingham; R. Tucker, Wenatchee, Wash; S. Tyring, Nassau Bay, Tex; M. Vranian, Richmond, Va; K. Williams, Saskatoon, Saskatchewan; and N. Zaias, Miami Beach, Fla.
Corresponding author: Stephen K. Tyring, MD, PhD, Rte 1070, University of Texas Medical Branch, Galveston, TX 77555.
From the Department of Dermatology, University of Texas Medical Branch at Galveston (Dr Tyring); Solano Dermatology Associates, Vallejo, Calif (Dr Beutner); Simon-Williamson Clinic PC, Birmingham, Ala (Dr Tucker) and Glaxo Wellcome Research and Development, Greenford, England (Dr Crooks). Dr Anderson is in private practice in New Braunfels, Tex. Drs Tyring and Beutner are consultants to and members of a speakers' program involving Glaxo Wellcome Inc, Research Triangle Park, NC.
REFERENCES
 |  |
1. Whitley RJ, Kroon S, Wood MJ, Sandstrom E. Guidelines and Recommendations From the Inaugural Meeting of the IHMF. Worthing, England: PPS Europe; 1993.
2. Burgoon CF, Burgoon JS, Baldridge GD. The natural history of herpes zoster. JAMA. 1957;164:265-269.
ISI
3. Hope-Simpson RE. The nature of herpes zoster: a long-term study and a new hypothesis. Proc R Soc Med. 1965;58:9-20.
ISI
| PUBMED
4. Bhala BB, Ramamorthy C, Bowsher D. Shingles and post-herpetic neuralgia. Clin J Pain. 1988;4:169-175.
5. Davies L, Cossins L, Bowsher D, Drummond M. The cost of treatment for post-herpetic neuralgia in the UK. Pharmacoeconomics. 1994;6:142-148.
ISI
| PUBMED
6. Beutner K, Friedman D, Forszpaniak C, et al. Valaciclovir compared with acyclovir for improved therapy for herpes zoster in immunocompetent adults. Antimicrob Agents Chemother. 1995;39:1546-1553.
ABSTRACT
7. Tyring S, Barbarash RA, Nahlik JE, et al. Famciclovir for the treatment of acute herpes zoster: effects on acute disease and post-herpetic neuralgia. Ann Intern Med. 1995;123:89-96.
FREE FULL TEXT
8. Standring-Cox R, Bacon TH, Howard B, Gilbart J, Boyd MR. Prolonged activity of penciclovir in cell culture against varicella-zoster virus [abstract]. Antiviral Res. 1994;23(suppl 1):96.
9. Griffiths PD. Spectrum of activity of antiherpesvirus drugs. Antivir Chem Chemother. 1994;5(suppl 1):17-22.
10. Grant DM, Mauskopf J, Bell L, Austin R. Comparison of valaciclovir and acyclovir for the treatment of herpes zoster in immunocompetent patients over 50 years of age: a cost consequence model. Pharmacotherapy. 1997;17:333-341.
ISI
| PUBMED
11. Huse DM, Schainbaum S, Kirsch AJ, Tyring S. Economic evaluation of famciclovir in reducing the duration of postherpetic neuralgia. Am J Health Syst Pharm. 1997;54:1180-1184.
FREE FULL TEXT
12. Degreef H. Famciclovir, a new oral antiherpes drug: results of the first controlled clinical study demonstrating its efficacy and safety in the treatment of uncomplicated herpes zoster in immunocompetent patients. Int J Antimicrob Agents. 1994;4:241-246.
13. Hope-Simpson RE. Postherpetic neuralgia. J R Coll Gen Pract. 1975;25:571-575.
PUBMED
14. De Moragas JM, Kierland RR. The outcome of patients with herpes zoster. Arch Dermatol. 1957;75:193-196.
FREE FULL TEXT
15. Wood MJ, ed, Kroon S, ed. Management Strategies in Herpes: Reducing the Burden of Zoster-Associated Pain—Update. Worthing, England: PPS Europe; 1996.
16. Famvir [package insert]. Philadelphia, Pa: SmithKline Beecham Pharmaceuticals; 2000.
17. Freedman LS. Tables of the number of patients required in clinical trials using the logrank test. Stat Med. 1982;1:121-129.
PUBMED
18. Kalbfleisch JD, Prentice RL. The Analysis of Failure-Time Data. New York, NY: John Wiley & Sons Inc; 1978.
19. Cox DR. Regression models and life table. J R Stat Soc B. 1972;34:187-220.
20. Whitley RJ, Shukla S, Crooks RJ. The identification of risk factors associated with persistent pain following herpes zoster. J Infect Dis. 1998;178(suppl 1):S71-S75.
21. Dworkin RH, Portenoy RK. Proposed classification of herpes zoster pain [letter]. Lancet. 1994;343:1648.
22. Wood MJ the Herpes Zoster Clinical Trial Consensus Group. For debate: how should zoster trials be conducted? J Antimicrob Chemother. 1995;36:1089-1101.
FREE FULL TEXT
23. Dworkin RH, Carrington D, Cunningham A, et al. Assessment of pain in herpes zoster: lessons learned from antiviral trials. Antiviral Res. 1997;33:73-85.
FULL TEXT
|
ISI
| PUBMED
24. Dworkin RH, Hartstein G, Rosner HL, Walther RR, Sweeney EW, Brand L. A high-risk method for studying psychosocial antecedents of chronic pain: the prospective investigation of herpes zoster. J Abnorm Psychol. 1992;101:200-205.
FULL TEXT
|
ISI
| PUBMED
25. Wood MJ, Kay R, Dworkin RH, Soong S-J, Whitley RJ. Oral acyclovir therapy accelerates pain resolution in patients with herpes zoster: a meta-analysis of placebo-controlled trials. Clin Infect Dis. 1996;22:341-347.
ISI
| PUBMED
26. Famvir [package insert]. Welwyn Garden City, England: SmithKline Beecham Pharmaceuticals PLL; 1999.
27. Famvir [package insert]. Dandenong, Victoria, Australia: SmithKline Beecham(Aust) Pharmaceuticals Pty Ltd; 1999.
28. Red Book 1999: Supplement April 1999. Montvale, NJ: Medical Economics Data; 1999.
RELATED ARTICLE
The Archives of Family Medicine Continuing Medical Education Program
Arch Fam Med. 2000;9(9):887-891.
FULL TEXT
THIS ARTICLE HAS BEEN CITED BY OTHER ARTICLES
 |
A 70-Year-Old Woman With Shingles: Review of Herpes Zoster
Whitley
JAMA 2009;302:73-80.
ABSTRACT
| FULL TEXT
Management Strategies for Herpes Zoster and Postherpetic Neuralgia
Galluzzi
JAOA: Journal of the American Osteopathic Association 2007;107:S8-S13.
ABSTRACT
| FULL TEXT
Herpes zoster and the prevention of postherpetic neuralgia: Beyond antiviral therapy
Tenser and Dworkin
Neurology 2005;65:349-350.
FULL TEXT
VZV vasculopathy and postherpetic neuralgia: Progress and perspective on antiviral therapy
Gilden et al.
Neurology 2005;64:21-25.
ABSTRACT
| FULL TEXT
80-Year-Old Man With Fever and Ear Pain
Lim and Takahashi
Mayo Clin Proc. 2004;79:1055-1058.
Risk factors for postherpetic neuralgia in patients with herpes zoster
Jung et al.
Neurology 2004;62:1545-1551.
ABSTRACT
| FULL TEXT
Herpes Zoster
Gnann and Whitley
NEJM 2002;347:340-346.
FULL TEXT
Valacyclovir or Famciclovir for Herpes Zoster?
Journal Watch Dermatology 2000;2000:7-7.
FULL TEXT
|