 |
 |

Picornavirus Infections
A Primer for the Practitioner
Harley A. Rotbart, MD;
Frederick G. Hayden, MD
Arch Fam Med. 2000;9:913-920.
ABSTRACT
Picornaviruses, including the rhinoviruses and enteroviruses, are common causes of infections in the developed world and the most common reason for prescribing antibiotics. These ubiquitous pathogens are increasingly being recognized in more serious illnesses, such as sinusitis, exacerbations of asthma, exacerbations of cystic fibrosis, myocarditis, meningitis, and severe neonatal sepsislike disease. Recent advances have improved our ability to diagnosis and treat these infections.
INTRODUCTION
The picornaviruses are a diverse group of human viral pathogens that together constitute the most common causes of infections of humans in the developed world. Within the picornavirus family, there are 3 well-known groups of human pathogens: the human rhinoviruses (HRVs); the enteroviruses (EVs) (including polioviruses, coxsackieviruses, and echoviruses); and the hepatoviruses (including hepatitis A). Hepatitis A, which differs significantly from the others genomically and clinically, has been recently reviewed elsewhere.1 This article will focus on the rhinoviruses and enteroviruses.
RHINOVIRUSES
The human rhinoviruses include more than 100 serotypes, in 2 main groups based on their cellular receptors. Human rhinoviruses cause about one half of all common colds,2 the leading cause of acute infectious morbidity worldwide; it is now also known to directly or indirectly cause many related respiratory ailments (Table 1). In addition, HRV infections cause lower respiratory tract disease in select populations (Table 1). Human rhinoviruses are epidemic in fall and spring, but infections occur year round.
|
|

|
|
Clinical Illness Caused by Picornavirus Infections
|
|
|
Common colds and related syndromes are the most frequent reasons for antibiotic use in the United States.3-4 This excessive use undoubtedly contributes to the increasing prevalence of antibiotic resistance in pathogenic bacteria and emphasizes the need for effective prophylaxis and treatment of HRV infections.
Pathogenesis
The pathogenesis of HRV-induced illness is incompletely understood. In persons lacking specific immunity to the infecting serotype, most exposures result in infection. Symptoms may begin within 12 hours of infection.5 Transmission seems to be via hand-nose or hand-eye contact after contamination of the hand with nasal secretions from an infected index case. The initial event in cold production is viral infection of the nasal epithelium; however, the number of productively infected cells seems to be small6-7 and nasal biopsy studies show little mucosal damage.8 Elaboration of the patients' inflammatory cytokines in response to viral infection is central in the pathogenesis of symptoms and in inducing immune responses. Respiratory epithelial cytokine production likely contributes to airway hyperreactivity and to an influx of neutrophils in both nasal mucosa and secretions.9 Cold symptoms also seem to be caused by neurologic reflexes triggered by the infection.10 Human rhinovirus is assumed to be restricted to the upper respiratory tract, but limited evidence indicates that HRV sometimes replicates in the tracheobronchial tree and lung.11
Clinical Manifestations
The usual incubation period of HRV colds is 1 to 3 days. Rhinorrhea, nasal stuffiness, and sneezing are the commonest symptoms; other typical manifestations include sore or scratchy throat, facial pressure, headache, cough, hoarseness, and, less often, malaise, chills, or feverishness.12 Sore throat tends to be the first symptom, and runny nose the most bothersome.13 Significant fever is very uncommon in adults and should suggest an alternative diagnosis. Infants and young children have fever more often and may show only mucus nasal discharge. Red, sometimes macerated nostrils and glassy nasal mucosa are also typically present, but examination is primarily useful to exclude other diagnoses. In adults, nasal symptoms usually peak on the second or third day and then gradually subside. Cough usually persists until the end of the first week but may be protracted in smokers. The median duration of HRV colds is 1 week, but up to one quarter last more than 2 weeks.13
Human rhinovirus infections are associated with a number of upper and lower respiratory tract complications in both children and adults. Viral respiratory tract infections are the most important predisposing factor to acute otitis media (AOM). Viruses have been detected by culture or antigen assay in 11% to 41% of middle ear fluids from children with AOM,14-15 and HRV is found in up to 8% of such fluids. By polymerase chain reaction (PCR), HRV infection is detectable in 35% of children with AOM, including the presence of HRV RNA in 24% of middle ear fluids.16 In adults, middle ear pressure abnormalities commonly develop during HRV colds.17-18 Coinfection with HRV and bacteria has been reported to predispose to failure of antibiotic therapy in AOM.19
Most cases of acute sinusitis thought to result from bacterial disease are secondary to a preceding viral upper respiratory tract infection. Sinus abnormalities are frequently detectable during uncomplicated colds.20 Consequently, distinguishing a primary viral rhinosinusitis from a secondary bacterial sinusitis is clinically difficult. Human rhinovirus is detectable by culture or PCR in 40% of sinus brushings from patients with acute community-acquired sinusitis.21
Human rhinovirus infections are major factors in the induction of acute exacerbations of asthma in both adults22 and children.23 In a 2-year study of adults with asthma aged 19 to 46 years, peak expiratory flow rate deteriorations occurred during 27% of respiratory illness episodes, and colds were associated with 71% of documented exacerbations.22 Human rhinoviruses are the most commonly identified pathogens found in asthma exacerbations and hospitalizations for those older than 2 years.24
Human rhinovirus infections are also associated with lower respiratory tract syndromes in other patient populations. In children with cystic fibrosis, picornaviruses were detected in about one fifth of exacerbations and colds were associated with deterioration in pulmonary function testing.25 Among adults aged 60 to 90 years residing in the community, HRV infection was associated with lower respiratory tract symptoms in 65%; 40% consulted their physician, and 76% of these received antibiotics.26 The impact of HRVs in elderly people, as measured by those indices, approachs that of influenza.26 Up to 40% of exacerbations in patients with chronic bronchitis may be associated with HRV infections.27 In infants younger than 12 months, HRV infections have been associated with hospitalization for lower respiratory tract illness, particularly bronchiolitis,28 and deterioration in those with bronchopulmonary dysplasia.29
Diagnosis
No rapid antigen detection or practical serologic tests exist for HRV infections because of the multiplicity of serotypes. Viral culture takes 3 to 7 days and is of limited clinical use. Polymerase chain reaction detection of HRV RNA has been frequently positive in HRV culture–negative samples,30 but currently is only a research tool.
Treatment
Cold treatments are associated with subjectivity and strong placebo effects, so adequate blinding of studies is essential. It is difficult to draw positive or negative conclusions from many of these studies because of small sample sizes, limited microbiological support, and differing outcome measures. Recent examples of interventions shown to be of no real value include intranasal hyperthermia,31 zinc gluconate lozenges,32 and oral Echinacea.33
Antivirals
A major hurdle to effective antiviral therapy is the unclear importance of ongoing viral replication in symptom pathogenesis after illness onset. The only agent consistently shown to have prophylactic activity against HRV infections is intranasal interferon.34 Combinations of intranasal interferon, intranasal ipratropium, and oral naproxen provide significantly greater clinical benefits than monotherapy in experimental colds.35 Such results support the general concept of treating HRV colds with combinations of antiviral and anti-inflammatory drugs.
A recently developed drug, pleconaril, inhibits viral replication by blocking viral uncoating and viral attachment to host-cell receptors.36 Pleconaril has demonstrated potent, broad-spectrum, anti-HRV activity and is highly orally bioavailable.37 Oral pleconaril was protective against experimental coxsackievirus A21 (an EV that behaves biologically like the HRVs) upper respiratory infection in volunteers, as reported in an abstract.38 In a double-blind, placebo-controlled study of 1024 adults with viral respiratory infection during the fall rhinovirus season, patients receiving pleconaril recovered from all cold symptoms and returned to overall wellness (measured via a global assessment score) 3.5 days sooner than patients receiving placebo, as reported in an abstract.39 Individual symptoms of the cold each resolved 1 or 2 days sooner in the pleconaril-treated patients. There were no differences in adverse effects between treatment and placebo groups.39 Studies of this drug in other picornavirus diseases (see the "Treatment" subsection of the "Enteroviruses" section) indicate both a clinical and virologic beneficial effect. Studies in HRV exacerbations of asthma and otitis media prevention are under way. Other molecules that inhibit protease enzyme activity of rhinoviruses are in development but have not yet reached clinical efficacy trials.
Symptomatic Therapies
Antihistamines have been frequently used for the treatment of common colds but their usefulness has been the subject of controversy.40 Only first-generation antihistamines (eg, chlorpheniramine, clemastine), which have anticholinergic and sedating effects, are useful in treating cold-associated rhinorrhea and sneezing.41-42 Selective, nonsedating second-generation antihistamines (eg, terfenadine, loratadine) are ineffective.43 The anticholinergic nasal spray ipratropium bromide has been shown to reduce rhinorrhea by 30% in natural colds.44 Corticosteroids do not provide clinically meaningful benefit in HRV colds and may serve to increase viral replication.45 Nonsteroidal anti-inflammatory agents variably benefit cold symptoms but certain ones (eg, ibuprofen, naproxen) relieve discomfort and systemic symptoms.46
Antibiotics
Despite their frequent use, no convincing evidence of benefit exists for antibiotic use during colds. A recent meta-analysis47 found that antibiotic treatment in more than 1500 children did not affect the symptoms of the common cold. A subset of about 20% of cold sufferers are colonized with pathogenic respiratory bacteria (Streptococcus pneumoniae, Haemophilus influenzae, Moraxella catarrhalis) and may experience modest symptom benefit and lower rates of subsequent antibiotic use if treated with amoxicillin clavulanate.48 Noncolonized patients do not benefit, and gastrointestinal intolerance develops 5-fold more often in amoxicillin-clavulanate recipients.48 Determination of carriers of pathogenic bacteria is not practical in the office setting and of doubtful clinical value. Antibiotics should be withheld unless secondary bacterial infections are strongly suspected.
ENTEROVIRUSES
The EVs include nearly 70 serotypes of closely related pathogens that cause a wide spectrum of illness (Table 1). The EVs are epidemic every summer and fall but, like HRVs, sporadic infections occur year round.
Pathogenesis
Enteroviruses are acquired by fecal-oral contamination and, less commonly, by respiratory droplet.49 Some replication occurs in the nasopharynx, but most of the viral inoculum is swallowed and infects the gastrointestinal tract. Unlike HRVs, the acid stability of the EVs allows them to traverse the stomach and reach the Peyer patches in the lower gastrointestinal tract, where the EVs replicate. A minor viremia ensues, which may seed numerous organ systems, including the central nervous system, liver, lungs, and heart. Further replication at these sites results in a major viremia associated with clinical manifestations.49
The patient's immune system and genetic background contribute to EV pathogenesis. Myocarditis, for example, represents an intricate interplay between virus and patient responses, in which both direct viral injury and immunopathologic damage caused by innocent-bystander phenomena affect the disease course.50 The EVs are cleared from the patient largely by antibody-mediated mechanisms. Agammaglobulinemic individuals infected with the EVs may develop chronic meningoencephalitis lasting many years, often with a fatal outcome.51
Clinical Manifestations
Nonspecific Febrile Illnesses
It is estimated that between 10 and 15 million people in the United States annually develop minor EV infections, characterized by fever and constitutional symptoms, with or without rashes.52 These illnesses are of clinical significance because they may mimic other diseases, including bacterial sepsis, other viral exanthematous diseases, and herpes simplex infections. The patients most affected by this mimicry are young infants, in whom differentiation of viral illness from more alarming causes of fever and rash is extremely difficult. One prospective study found that 13% of babies born in the summer months were infected with EVs during the first month of life; 21% of the infected babies were hospitalized for suspected bacterial sepsis and received antibiotics or antiherpes therapy.53 Manifestations include fever (temperature usually 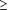 39°C), irritability, lethargy, anorexia, diarrhea, vomittng, rash, and respiratory symptoms. The duration of illness in infants beyond the neonatal period is usually 4 to 5 days. 39°C), irritability, lethargy, anorexia, diarrhea, vomittng, rash, and respiratory symptoms. The duration of illness in infants beyond the neonatal period is usually 4 to 5 days.
Respiratory Illnesses
Many EV infections are accompanied by nonspecific, usually mild, respiratory illness. A recent review of EV-associated respiratory illnesses found that 46% of cases presented with upper respiratory infections, 13% with respiratory distress/apnea, 13% with pneumonia, and 12% with otitis media; others presented with bronchiolitis, wheezing, croup, and pharyngotonsillitis.54 Most EV-associated respiratory illnesses are indistinguishable from those caused by other respiratory viruses. Several distinctive syndromes are described below.
Hemorrhagic Conjunctivitis
After an incubation period of about 24 hours, hemorrhagic conjunctivitis is characterized by rapid onset of swelling of the eyelids, with congestion, lacrimation, and pain. Epithelial keratitis is common and transient. Some patients develop subconjunctival hemorrhages. In outbreak situations, incidence figures may reach pandemic proportions and neurologic complications, including paralytic poliolike disease, have been reported.55
Herpangina
The highest incidence of herpangina is among children aged 1 to 7 years, but infection has also been described in both neonates and adults.56 Abrupt onset of fever associated with a sore throat, dysphagia, and malaise is typical. One fourth of the patients present with vomiting and abdominal pain. Early in the illness, grayish-white vesicles measuring 1 to 4 mm in diameter appear over the posterior portion of the palate uvula, on tonsillar pillars, and, occasionally, on the oropharynx. These vesicles are discrete, surrounded by erythema, and are usually fewer than 20. Symptoms begin to improve in 4 to 5 days, and recovery is usually complete within a week of onset.56
Hand-Foot-Mouth Syndrome
Hand-foot-mouth syndrome typically occurs among children younger than 4 years, but adults are also frequently affected; intrafamilial spread is common. The disease is usually mild and the onset is associated with a sore throat with or without a low-grade fever.57 Scattered vesicular lesions occur randomly on the oral structures, the pharynx, and the lips; these ulcerate readily, leaving shallow lesions with red areolae. Sparse grayish vesicles (3-5 mm in diameter, surrounded by erythema) also appear on the dorsum of the fingers, particularly in periungal areas, and on the margins of heels; palmar, plantar, and groin lesions may also appear. As with hemorrhagic conjunctivitis, massive outbreaks of hand-foot-mouth syndrome have been reported, with prominent neurologic and other serious systemic manifestations.58
Pleurodynia
Pleurodynia is primarily a disease of muscle masquerading as pleuritic disease, although pleural involvement can occur.59 The onset is abrupt in three fourths of patients, with the remainder first developing headache and other vague prodromal symptoms of 1 to 10 days in duration. The major symptom is severe paroxysmal pain referred to the lower ribs or the sternum; however, fever, headache, cough, anorexia, nausea, vomiting, and diarrhea also can occur. The mean duration of the illness is 3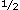 days, varying from 1 to 14 days.59 days, varying from 1 to 14 days.59
Myocarditis
The EVs are among the most commonly identified origins of myocarditis, causing between 25% and 35% of cases for which a cause is found.50 Neonates and infants ( 6 months of age) are particularly susceptible to EV myocarditis (see the "Congenital and Neonatal Infections" subsection), but most cases occur in young adults between the ages of 20 and 39 years. Rigorous exercise and recent respiratory illness are anecdotally associated with many cases of myocarditis. Clinically, myocarditis reflects the extent of the cardiac involvement50; symptoms include palpitations and chest pain, often with accompanying fever. Arrhythmias and sudden death reflect conducting system involvement, often of very recent onset; congestive heart failure or myocardial infarction–like presentations suggest more extensive myocyte necrosis and longer-standing disease. Pericardial friction rub indicates a myopericarditis. Electrocardiographic findings include an evolution from early-stage ST-segment elevation and T-wave inversion to intermediate stage normalization to late-stage recurrence of T-wave inversion. Myocardial enzyme elevations are detected in the blood. While most patients recover uneventfully from clinically apparent myocarditis, many have residual electrocardiographic or echocardiographic abnormalities for months to years. Smaller percentages of patients develop congestive heart failure, chronic myocarditis, or dilated cardiomyopathy.50 6 months of age) are particularly susceptible to EV myocarditis (see the "Congenital and Neonatal Infections" subsection), but most cases occur in young adults between the ages of 20 and 39 years. Rigorous exercise and recent respiratory illness are anecdotally associated with many cases of myocarditis. Clinically, myocarditis reflects the extent of the cardiac involvement50; symptoms include palpitations and chest pain, often with accompanying fever. Arrhythmias and sudden death reflect conducting system involvement, often of very recent onset; congestive heart failure or myocardial infarction–like presentations suggest more extensive myocyte necrosis and longer-standing disease. Pericardial friction rub indicates a myopericarditis. Electrocardiographic findings include an evolution from early-stage ST-segment elevation and T-wave inversion to intermediate stage normalization to late-stage recurrence of T-wave inversion. Myocardial enzyme elevations are detected in the blood. While most patients recover uneventfully from clinically apparent myocarditis, many have residual electrocardiographic or echocardiographic abnormalities for months to years. Smaller percentages of patients develop congestive heart failure, chronic myocarditis, or dilated cardiomyopathy.50
Aseptic Meningitis
The severity of EV meningitis varies with patient age and immune status.60 Neonates younger than 2 weeks are at risk for severe systemic illness (see the "Congenital and Neonatal Infections" subsection), commonly including meningitis or meningoencephalitis. Enterovirus meningitis beyond the first 2 weeks of life is rarely associated with severe disease or poor outcome. Onset is usually sudden, and a temperature of 38° to 40°C occurs in 75% to 100% of patients.60 The fever pattern may be biphasic, appearing first with nonspecific constitutional symptoms, and reappearing with the onset of meningeal signs. Headache is nearly always present in adults and children old enough to report it, and photophobia is also common.61 Nuchal rigidity is found in more than half of the patients, particularly in children older than 1 or 2 years. Nonspecific manifestations include vomiting, anorexia, rash, diarrhea, cough and pharyngitis, diarrhea, and myalgias. Neurologic abnormalities are unusual. The duration of illness caused by EV meningitis is usually about 1 week, but many patients, particularly adults, may have symptoms that persist for 2 or more weeks.61 The prognosis for young children with EV meningitis early in life seems to be good, without long-term sequelae.62
Encephalitis
Encephalitis caused by the EVs is well documented but uncommon.63 Unlike aseptic meningitis, encephalitis caused by the EVs may have more profound acute disease and long-term sequelae. In contrast to the typical focal disease seen with herpes simplex virus, EVs are usually associated with global encephalitis and generalized neurologic depression. The illness usually begins with a prodrome of fever, myalgias, and upper respiratory symptoms. Onset of central nervous system signs and symptoms is often abrupt, with confusion, weakness, lethargy, drowsiness, and/or irritability; progression to coma and/or generalized seizures may occur. Focal EV encephalitis is less often reported than global disease but may be underappreciated.64
Congenital and Neonatal Infections
Neonates seem to be at greatest risk for severe disease when infection and illness develop in the first days of life; this pattern suggests possible transplacental acquisition.65 Maternal illness has been reported in 59% to 68% of infected neonates. Fever is ubiquitous, often accompanied by vomiting, anorexia, rash, and/or upper respiratory findings. Neurologic involvement may or may not be associated with signs of meningeal inflammation, including nuchal rigidity and a bulging anterior fontanelle. Major systemic manifestations, such as hepatic necrosis, myocarditis, and necrotizing enterocolitis, may develop. Disseminated intravascular coagulation and other findings of sepsis result in illness, which may be indistinguishable from overwhelming bacterial infection. An encephalitic picture with seizures and focal neurologic findings may suggest herpes simplex virus. As many as 2500 cases of neonatal EV sepsis may occur each year; the incidences of severe morbidity and mortality occurring with perinatal EV infections are not precisely known, but may be as high as 74% and 10%, respectively.66-67 Mortality is typically because of hepatic failure or myocarditis.
Diagnosis
Isolation of EVs in cell culture has been the criterion standard for laboratory diagnosis for many years.68 However, no single cell line is optimal for all EV serotypes, and virus isolation requires technical expertise and may be quite labor intensive. Additionally, 25% to 35% of specimens from patients with EV infections will be falsely negative by cell culture.
The most promising development in detection of the EVs has been PCR, which is consistently more sensitive than culture and virtually 100% specific.69 This assay is available from numerous reference laboratories around the country. The most appropriate specimens for testing depend on the syndrome: cerebrospinal fluid for meningitis and encephalitis, serum and urine for neonatal sepsis, and throat swab for respiratory illness. The rapidity of this assay (5 hours in some formats), coupled with its high degree of accuracy, have made PCR the test of choice for EV diagnosis at centers that offer it.
Treatment
Immune Serum Globulin
Immune serum globulin (ISG) has been used prophylactically and therapeutically against EVs in 2 patient groups: neonates and immunocompromised patients. Anecdotal reports of clinical success with maternal serum or plasma, or commercial ISG, against a variety of EV serotypes causing severe neonatal EV disease have been reported; other reports describe progressive disease and death despite such therapy.65 One small controlled study demonstrated reduction of viral titers in babies receiving intravenous ISG preparations subsequently shown to contain high antibody titers to the infecting serotype.70
Before the availability of intravenous ISG, mixed results were reported with intramuscular and/or intrathecal administration of ISG in patients with antibody deficiencies.51 Since patients with known antibody deficiencies began receiving prophylactic intravenous ISG, the incidence of progressive EV meningoencephalitis has fallen and the clinical profile of patients developing such infections has been modified.71 However, it remains unclear if intravenous ISG has therapeutic efficacy in established EV meningoencephalitis in these patients.
Pleconaril
Pleconaril has broad anti-EV activity as well as anti-HRV activity, as noted above. Potent systemic activity of pleconaril has been demonstrated in volunteers with experimental coxsackievirus A21 respiratory infections38 and in compassionate-use treatment of potentially life-threatening EV infections, as reported in an abstract.72 In a placebo-controlled trial of pleconaril in 221 pediatric patients with EV meningitis, significant reductions in the total morbidity (composite measurement of all disease symptoms) and global assessment (caregivers' assessment of patients' illness) scores were documented for the overall study population, as reported in an abstract.73 Headache duration was significantly reduced by pleconaril treatment in children older than 8 years. Responses were noted as early as 24 hours after initiation of treatment. Viral shedding from the throat, reflecting duration of infection, was also reduced in the pleconaril-treated group compared with placebo.73 Pleconaril has also been studied in adult patients with EV meningitis and reported in an abstract.74 In a double-blind, placebo-controlled trial, 180 patients aged 14 to 65 years received either 200 mg of pleconaril 3 times per day or placebo. Those receiving pleconaril had a 2-day shorter duration of headache and a 2-day faster resolution of all symptoms of meningitis. Pleconaril-treated patients also returned to work or school 2 days faster.74
Pleconaril is in phase 3 clinical trials for both meningitis and viral respiratory infections caused by the picornaviruses. It is available by compassionate use for the sickest patients with confirmed or highly suspected picornavirus diseases. Food and Drug Administration application is anticipated within the coming 12 to 18 months.
CONCLUSIONS
The picornaviruses, including the EVs and HRVs, are the most common causes of viral illnesses worldwide. Ranging from mild diseases of short duration to severe and potentially life-threatening infections, the picornaviruses have protean clinical presentations. New developments in the rapid diagnosis and therapy of these infections may reduce the disease burden and the associated costs to affected individuals and to society.
AUTHOR INFORMATION
Accepted for publication November 24, 1999.
Corresponding author: Harley A. Rotbart, MD, University of Colorado Health Sciences Center, 4200 E Ninth Ave, Box C227, Denver, CO 80262 (e-mail: harley.rotbart{at}uchsc.edu).
From the Departments of Pediatrics and Microbiology, University of Colorado Health Sciences Center, Denver (Dr Rotbart), and the Department of Medicine, University of Virginia Health Sciences Center, Charlottesville (Dr Hayden).
REFERENCES
 |  |
1. Koff RS. Hepatitis A. Lancet. 1998;351:1643-1649.
FULL TEXT
|
ISI
| PUBMED
2. Makela MJ, Puhakka T, Ruuskanen O, et al. Viruses and bacteria in the etiology of the common cold. J Clin Microbiol. 1998;36:539-542.
FREE FULL TEXT
3. Gonzales R, Steiner JF, Sande MA. Antibiotic prescribing for adults with colds, upper respiratory tract infections, and bronchitis by ambulatory care physicians. JAMA. 1997;278:901-904.
FREE FULL TEXT
4. Nyquist AC, Gonzales R, Steiner JF, Sande MA. Antibiotic prescribing for children with colds, upper respiratory tract infections, and bronchitis. JAMA. 1998;279:875-877.
FREE FULL TEXT
5. Harris JM, Gwaltney JM Jr. Incubation periods of experimental rhinovirus infection and illness. Clin Infect Dis. 1996;23:1287-1290.
ISI
| PUBMED
6. Bardin PG, Johnston SL, Sanderson G, et al. Detection of rhinovirus infection of the nasal mucosa by oligonucleotide in situ hybridization. Am J Respir Cell Mol Biol. 1994;10:207-213.
ABSTRACT
7. Arruda E, Boyle TR, Winther B, Pevear DC, Gwaltney JM, Hayden FG. Localization of human rhinovirus replication in the upper respiratory tract by in situ hybridization. J Infect Dis. 1995;171:1329-1333.
ISI
| PUBMED
8. Winther B. Effects on the nasal mucosa of upper respiratory viruses (common cold). Dan Med Bull. 1994;41:193-204.
ISI
| PUBMED
9. Turner RB, Weingand K, Yeh CH, et al. Association between interleukin-8 (IL-8) and symptom severity in rhinovirus colds. In: Proceedings of the 36th Interscience Conference on Antimicrobial Agents and Chemotherapy; September 15-18, 1996; New Orleans, La.
10. Igarashi Y, Skoner DP, Doyle WJ. Analysis of nasal secretions during experimental rhinovirus upper respiratory infections. J Allergy Clin Immunol. 1993;92:722-731.
FULL TEXT
|
ISI
| PUBMED
11. Shelhamer JH, Levine SJ, Wu T, Jacoby DB, Kaliner MA, Rennard SI. NIH conference: airway inflammation. Ann Intern Med. 1995;123:288-304.
FREE FULL TEXT
12. Gwaltney JM Jr, Rueckert RR. Rhinovirus. In: Richman DD, Whitley RJ, Hayden FG, eds. Clinical Virology. New York, NY: Churchill Livingstone; 1997:1025-1047.
13. Arruda E, Pitkaranta A, Witek TJ Jr, Doyle CA, Hayden FG. Frequency and natural history of rhinovirus infections in adults during autumn. J Clin Microbiol. 1997;35:2864-2868.
ABSTRACT
14. Heikkinen T, Thint M, Chonmaitree T. Prevalence of various respiratory viruses in the middle ear during acute otitis media. N Engl J Med. 1999;340:260-264.
FREE FULL TEXT
15. Arola M, Ruuskanen O, Ziegler T, et al. Clinical role of respiratory virus infection in acute otitis media. Pediatrics. 1990;86:848-855.
FREE FULL TEXT
16. Pitkaranta A, Virolainen A, Jero J, Arruda E, Hayden FG. Detection of rhinovirus respiratory syncytial virus and coronavirus infections in acute otitis media by reverse transcriptase polymerase chain reaction. Pediatrics. 1998;102(2, pt 1):291-295.
17. Buchman CA, Doyle WJ, Skoner D, Fireman P, Gwaltney JM. Otologic manifestations of experimental rhinovirus infection. Laryngoscope. 1994;104:1295-1299.
ISI
| PUBMED
18. Elkhatieb A, Hipskind G, Woerner D, Hayden FG. Middle ear abnormalities during natural rhinovirus colds in adults. J Infect Dis. 1993;168:618-621.
ISI
| PUBMED
19. Patel JA, Reisner B, Vizirinia N, Owen M, Chonmaitree T, Howie V. Bacteriological failure of amoxicillin-clavulanate in treatment of acute otitis media caused by nontypeable Haemophilus influenzae. J Pediatr. 1995;126:799-806.
FULL TEXT
|
ISI
| PUBMED
20. Gwaltney JM Jr, Phillips CD, Miller RD, Riker DK. Computed tomographic study of the common cold. N Engl J Med. 1994;330:25-30.
FREE FULL TEXT
21. Pitkaranta A, Arruda E, Malmberg H, Hayden FG. Detection of rhinovirus in sinus brushings of patients with acute community-acquired sinusitis by reverse transcription-PCR. J Clin Microbiol. 1997;35:1791-1793.
ABSTRACT
22. Nicholson KG, Kent J, Ireland DC. Respiratory viruses and exacerbations of asthma in adults. BMJ. 1993;307:982-986.
23. Johnston SL, Pattemore PK, Sanderson G, Smith S, Lampe F, Josephs L. Community study of role of viral infections in exacerbations of asthma in 9-11 year old children. BMJ. 1995;310:1225-1229.
FREE FULL TEXT
24. Johnston SL, Pattemore PK, Sanderson G, et al. The relationship between upper respiratory infections and hospital admissions for asthma: a time-trend analysis. Am J Respir Crit Care Med. 1996;154(3, pt 1):654-660.
25. Collinson J, Nicholson KG, Gancio E, et al. Effects of upper respiratory tract infections in patients with cystic fibrosis. Thorax. 1996;51:1115-1122.
FREE FULL TEXT
26. Nicholson KG, Kent J, Hammersley V, Gancio E. Risk factors for lower respiratory complications of rhinovirus infections in elderly people living in the community: prospective cohort study. BMJ. 1996;313:1119-1123.
FREE FULL TEXT
27. Gwaltney JM Jr. Rhinoviruses. In: Evans AS, ed. Viral Infection of Humans. New York, NY: Plenum Publishing; 1989:593.
28. Schmidt HJ, Fink RJ. Rhinovirus as a lower respiratory pathogen in infants. Pediatr Infect Dis J. 1991;10:700-702.
FULL TEXT
29. Chidekel AS, Bazzy AR, Rosen CL. Rhinovirus infection associated with severe lower respiratory tract illness and worsening lung disease in infants with bronchopulmonary dysplasia. Pediatr Pulmonol. 1994;18:261-263.
ISI
| PUBMED
30. Gilbert LL, Dakhama A, Bone BM, Thomas EE, Hegele RG. Diagnosis of viral respiratory tract infections in children by using reverse transcription-PCR panel. J Clin Microbiol. 1996;34:140-143.
ABSTRACT
31. Hendley JO, Abbott RD, Beasley PP, Gwaltney JM Jr. Effect of inhalation of hot humidified air on experimental rhinovirus infection. JAMA. 1994;271:1112-1113.
FREE FULL TEXT
32. Mossad SB, Macknin ML, Medendorp SV, Mason P. Zinc gluconate lozenges for treating the common cold: a randomized, double-blind, placebo-controlled study. Ann Intern Med. 1996;125:81-88.
FREE FULL TEXT
33. Grimm W, Muller HH. A randomized controlled trial of the effect of fluid extract of Echinacea purpurea on the incidence and severity of colds and respiratory infections. Am J Med. 1999;106:138-143.
FULL TEXT
|
ISI
| PUBMED
34. Arruda E, Hayden FG. Clinical studies of antiviral agents for picornaviral infections. In: Jeffries DJ, DeCierrq E, eds. Antiviral Chemotherapy. New York, NY: John Wiley & Sons Inc; 1995:243-267.
35. Gwaltney JM Jr. Combined antiviral and antimediator treatment of rhinovirus colds. J Infect Dis. 1992;166:776-782.
ISI
| PUBMED
36. Rotbart HA, O'Connell JF, McKinlay MA. Treatment of human enterovirus infections. Antiviral Res. 1998;38:1-14.
FULL TEXT
|
ISI
| PUBMED
37. Pevear DC, Tull TM, Seipel ME, Groarke JM. Activity of pleconaril against enteroviruses. Antimicrob Agents Chemother. 1999;43:2109-2115.
FREE FULL TEXT
38. Schiff GM, McKinlay MA, Sherwood JR. Oral efficacy of VP 63843 in coxsackievirus A21 infected volunteers. In: Proceedings of the 36th Interscience Conference on Antimicrobial Agents and Chemotherapy; September 15-18, 1996; New Orleans, La. Abstract H-43.
39. Hayden FG, Hassman HA, Coats T, et al. Pleconaril treatment shortens duration of picornavirus respiratory illness in adults. In: Proceedings of the 39th Interscience Conference on Antimicrobial Agents and Chemotherapy; September 26-29, 1999; San Francisco, Calif. Abstract LB-3.
40. Luks D, Anderson MR. Antihistamines and the common cold: a review and critique of the literature. J Gen Intern Med. 1996;11:240-244.
ISI
| PUBMED
41. Gwaltney JM Jr, Park J, Paul RA, Edelman DA, O'Connor RR, Turner RB. Randomized controlled trial of clemastine fumarate for treatment of experimental rhinovirus colds. Clin Infect Dis. 1996;22:656-662.
ISI
| PUBMED
42. Turner RB, Sperber SJ, Sorrentino JV, et al. Effectiveness of clemastine fumarate for treatment of rhinorrhea and sneezing associated with the common cold. Clin Infect Dis. 1997;25:824-830.
ISI
| PUBMED
43. Berkowitz RB, Tinkelman DG. Evaluation of oral terfenadine for treatment of the common cold. Am Rev Resp Dis. 1987;136:556-560.
ISI
| PUBMED
44. Hayden FG, Diamond L, Wood PB, Korts DC, Wecker MT. Effectiveness and safety of intranasal ipratropium bromide in common colds: a randomized, double-blind, placebo-controlled trial. Ann Intern Med. 1996;125:89-97.
FREE FULL TEXT
45. Gustafson LM, Proud D, Hendley O, Hayden FG, Gwaltney JM. Oral prednisone therapy in experimental rhinovirus infections. J Allergy Clin Immunol. 1996;97:1009-1114.
FULL TEXT
|
ISI
| PUBMED
46. Sperber SJ, Hendley JO, Hayden FG, Riker DK, Sorrentino JV, Gwaltney JM Jr. Effects of naproxen on experimental rhinovirus colds: a randomized, double-blind, controlled trial. Ann Intern Med. 1992;117:37-41.
47. Gadomski AM. Potential interventions for preventing pneumonia among young children: lack of effect of antibiotic treatment for upper respiratory infections. Pediatr Infect Dis J. 1993;12:115-120.
ISI
| PUBMED
48. Kaiser L, Lew D, Hirschel B, et al. Effects of antibiotic treatment of the subset of common-cold patients who have bacteria in nasopharyngeal secretions. Lancet. 1996;347:1507-1510.
FULL TEXT
|
ISI
| PUBMED
49. Rotbart HA, Kirkegaard K. Picornavirus pathogenesis: viral access, attachment, and entry into susceptible cells. Semin Virol. 1992;3:483-499.
50. Martino TA, Liu P, Petric M, Sole MJ. Enteroviral myocarditis and dilated cardiomyopathy: a review of clinical and experimental studies. In: Rotbart HA, ed. Human Enterovirus Infections. Washington, DC: ASM Press; 1995:291-351.
51. McKinney RE Jr, Katz SL, Wilfert GM. Chronic enteroviral meningoencephalitis in agammaglobulinemic patients. Rev Infect Dis. 1987;9:334-356.
ISI
| PUBMED
52. Strikas RA, Anderson LJ, Parker RA. Temporal and geographic patterns of isolates of nonpolio enterovirus in the United States, 1970-1983. J Infect Dis. 1986;153:346-351.
ISI
| PUBMED
53. Jenista JA, Powell KR, Menegus MA. Epidemiology of neonatal enterovirus infection. J Pediatr. 1984;104:685-690.
ISI
| PUBMED
54. Chonmaitree T, Mann L. Respiratory infections. In: Rotbart HA, ed. Human Enterovirus Infections. Washington, DC: ASM Press; 1995:255-270.
55. Wadia NH, Wadia PN, Katrak SM, Misra VP. A study of the neurological disorder associated with acute hemorrhagic conjunctivitis due to enterovirus 70. J Neurol Neurosurg Psychiatry. 1983;46:599-610.
FREE FULL TEXT
56. Parrott RH, Ross S, Burke FG, Rice EC. Herpangina: clinical studies of a specific infectious disease. N Engl J Med. 1951;245:275-280.
57. Cherry JD. Enteroviruses: coxsackieviruses, echoviruses, and polioviruses. In: Feigin RD, Cherry JD, eds. Textbook of Pediatric Infectious Diseases. 4th ed. Philadelphia, Pa: WB Saunders Co; 1998:1787-1838.
58. Ho M, Chen ER, Hsu KH, et al for the Taiwan Enterovirus Epidemic Working Group. An epidemic of enterovirus 71 infection in Taiwan. N Engl J Med. 1999;341:929-935.
FREE FULL TEXT
59. Kantor FS, Hsiung GD. Pleurodynia associated with echo virus type 8. N Engl J Med. 1962;266:661-663.
60. Rotbart HA. Viral meningitis and the aseptic meningitis syndrome. In: Scheld WM, Whitley RJ, Durack DT, eds. Infections of the Central Nervous System. 2nd ed. Philadelphia, Pa: Lippincott-Raven Publishers; 1997:23-46.
61. Rotbart HA, Brennan PJ, Fife KH, et al. Enterovirus meningitis in adults. Clin Infect Dis. 1998;27:896-898.
ISI
| PUBMED
62. Rorabaugh ML, Berlin LE, Rosenberg L, Modlin J. Absence of neurodevelopmental sequelae from aseptic meningitis [abstract]. Pediatr Res. 1992;30:177A.
63. Whitley RJ, Cobbs CG, Alford CA Jr, et al for the NIAD Colloborative Antiviral Study Group. Diseases that mimic herpes simplex encephalitis: diagnoses, presentation, and outcome. JAMA. 1989;262:234-239.
FREE FULL TEXT
64. Modlin JF, Dagan R, Berlin LE, Virshup DM, Yolken RH, Menegus M. Focal encephalitis with enterovirus infections. Pediatrics. 1991;88:841-845.
FREE FULL TEXT
65. Abzug MJ, Levin MJ, Rotbart HA. Profile of enterovirus disease in the first two weeks of life. Pediatr Infect Dis J. 1993;12:820-824.
ISI
| PUBMED
66. Kaplan MH, Klein SW, McPhee J, Harper RG. Group B coxsackievirus infections in infants younger than three months of age: a serious childhood illness. Rev Infect Dis. 1983;5:1019-1032.
ISI
| PUBMED
67. Modlin JF. Perinatal echovirus infection: insights from a literature review of 61 cases of serious infection and 16 outbreaks in nurseries. Rev Infect Dis. 1986;8:918-926.
ISI
| PUBMED
68. Rotbart HA. Enteroviruses. In: Murray PR, Baron EJ, Pfaller MA, Tenover FC, Yolken RH, eds. Manual of Clinical Microbiology. 6th ed. Washington, DC: ASM Press; 1995:1004-1011.
69. Rotbart HA, Ahmed A, Hickey S, et al. Diagnosis of enterovirus infection by polymerase chain reaction of multiple specimen types. Pediatr Infect Dis J. 1997;16:409-411.
FULL TEXT
|
ISI
| PUBMED
70. Abzug MJ, Keyserling HL, Lee ML, Levin MJ, Rotbart HA. Neonatal enterovirus infection: virology, serology, and effects of intravenous immune globulin. Clin Infect Dis. 1995;20:1201-1206.
ISI
| PUBMED
71. Webster AD, Rotbart HA, Warner T, Rudge P, Hyman N. Diagnosis of enterovirus brain disease in hypogammaglobulinemic patients by polymerase chain reaction. Clin Infect Dis. 1993;17:657-661.
ISI
| PUBMED
72. Rotbart HA and the Pleconaril Treatment Registry. Pleconaril therapy of potentially life-threatening enterovirus infections. In: Abstracts of the 36th Annual Meeting of the Infectious Diseases Society of America; November 12-15, 1998; Denver, Colo. Abstract 791.
73. Sawyer MH, Saez-Llorenz X, Aviles CL, O'Ryan M, Romero J. Oral pleconaril reduces the duration and severity of enteroviral meningitis in children. In: Proceedings of the 1999 American Pediatric Society/Society for Pediatric Research Meetings; May 1-4, 1999; San Francisco, Calif.
74. Shafran SD, Halota W, Gilbert D, Bernstein J, Meislin H, Reiss M. Pleconaril is effective for enteroviral meningitis in adolescents and adults: a randomized placebo-controlled multicenter trial. In: Proceedings of the 39th Interscience Conference on Antimicrobial Agents and Chemotherapy; September 26-29; San Francisco, Calif. Abstract 1904.
RELATED ARTICLE
A Family Physician's Perspective on Picornavirus Infections in Primary Care
Jonathan L. Temte
Arch Fam Med. 2000;9(9):921-922.
FULL TEXT
THIS ARTICLE HAS BEEN CITED BY OTHER ARTICLES
Infection Rate and Virus-Induced Cytokine Secretion in Experimental Rhinovirus Infection in Mucosal Organ Culture: Comparison Between Specimens From Patients With Chronic Rhinosinusitis With Nasal Polyps and Those From Normal Subjects
Wang et al.
Arch Otolaryngol Head Neck Surg 2008;134:424-427.
ABSTRACT
| FULL TEXT
Diagnosis and Treatment of Rhinovirus Respiratory Infections
Anzueto and Niederman
Chest 2003;123:1664-1672.
ABSTRACT
| FULL TEXT
Respiratory Consequences of Rhinovirus Infection
Greenberg
Arch Intern Med 2003;163:278-284.
ABSTRACT
| FULL TEXT
A Family Physician's Perspective on Picornavirus Infections in Primary Care
Temte
Arch Fam Med 2000;9:921-922.
FULL TEXT
|