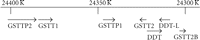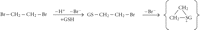Genetic Variations in Human Glutathione Transferase Enzymes: Significance for Pharmacology and Toxicology
- P. David Josephy djosephy{at}uoguelph.ca
Abstract
Glutathione transferase enzymes (GSTs) catalyze reactions in which electrophiles are conjugated to the tripeptide thiol glutathione. While many GST-catalyzed transformations result in the detoxication of xenobiotics, a few substrates, such as dihaloalkanes, undergo bioactivation to reactive intermediates. Many molecular epidemiological studies have tested associations between polymorphisms (especially, deletions) of human GST genes and disease susceptibility or response to therapy. This review presents a discussion of the biochemistry of GSTs, the sources—both genetic and environmental—of interindividual variation in GST activities, and their implications for pharmaco- and toxicogenetics; particular attention is paid to the Theta class GSTs.
1. Introduction: Pharmacogenomics and Personalized Medicine: A Perspective
The Golden Helix Symposium “Pharmacogenomics: paving the path to personalized medicine,” held in Athens in October 2009, brought together scientists and physicians who share the hope and expectation that molecular analysis of human genes affecting pharmacodynamics and pharmacokinetics will soon lead to significant medical advances. Several kinds of improvements can be anticipated. For example, starting drug doses may be tailored to an individual's metabolism, thereby increasing therapeutic efficacy and reducing side effects; individuals for whom a particular drug should be avoided altogether, to avert toxicity or “idiosyncratic” reactions, might be identified by prior genetic screening; and mechanistic insights into the development of particular diseases, drug side effects, or toxicities resulting from environmental exposures might be garnered by analysis of associations with specific genes [1, 2].
Our pursuit of this research agenda should be diligent but also balanced. Despite optimistic predictions, well-publicized in the popular press [3], clinical implementation of genetically guided drug therapy has been slow. Both fundamental and practical obstacles must be overcome before the clinical potential of pharmacogenomics is realized [4–6]. The goal of getting patients “the right drug in the right dose” must be kept in perspective; for many people, the urgent priority is to obtain any access at all to medical care and to authentic prescription drugs [7]. This article presents a review of the human glutathione transferases (GSTs) and their genes, in the context of pharmacogenetics and pharmacogenomics.
Many genetic polymorphisms affecting enzymes of xenobiotic metabolism strongly influence the pharmacokinetics of clinically-important drugs (e.g., warfarin and P450 2C9 [8], 6-mercaptopurine and thiopurine methyltransferase [9], irinotecan and UDP-glucuronosyltransferase 1A1 [10]). To date, there are no such clear cases with respect to GSTs. (The immunosuppressive drug azathioprine may prove to be one instance [11, 12].) This paucity of examples is certainly not due to a lack of genetic polymorphisms: GST polymorphisms are common and some of them have clear phenotypic consequences, as discussed below. Why, then, do GST polymorphisms apparently have less impact on pharmacokinetics? Several factors may be involved. First, GSTs catalyze detoxication of electrophilic compounds by conjugation to glutathione. Candidate drugs which give rise to substantial amounts of electrophilic reactive species at clinically effective doses are likely to be too toxic for use—the exception being cancer chemotherapeutic drugs [13–15], where electrophilic reactivity can be the mechanism of therapeutic action. Second, as discussed below, humans express a large number of different GSTs with overlapping substrate specificities, and the effects of polymorphisms (including gene deletions) affecting one GST may be masked by the activity of others. Third, in some cases where inactivation of a toxic drug metabolite by glutathione is critical for prevention of toxicity, such as the quinoneimine metabolite of acetaminophen, the nonenzymatic reaction may be fast enough that variations in enzyme activity are of little significance [16]. Fourth, genetic polymorphisms probably account for only a small proportion of the large interindividual variation in GST expression and activity [17–19]. Factors such as diet [20, 21], environmental chemical exposures [22], age [23], and gender [24], which remain only poorly understood, may be more important determinants. Nevertheless, our understanding of human GST polymorphisms is still limited, and clinical consequences may simply have gone unnoticed to date.
2. Glutathione Transferase Enzymes
2.1. Overview
Glutathione transferases (GSTs; systematically designated as “RX: glutathione R-transferases”, E.C. 2.5.1.18) are enzymes belonging to two protein superfamilies, the soluble GSTs and the “MAPEG” (Membrane Associated Proteins in Eicosanoid and Glutathione metabolism) proteins [5]. Soluble GSTs are dimers of 25 kDa subunits. Consequently, the homodimeric protein product of the GSTA1 gene, for example, is referred to as GST A1-1. Crystal structures have been determined for many soluble GSTs, often with bound substrates or products. The “canonical fold” of a soluble GST subunit reveals an N-terminal α/β domain forming the GSH-binding site (“G-site”) and a second, α-helical domain forming most of the “H-site” that binds the electrophilic substrate.
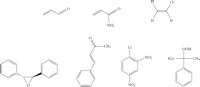
Isaiah Berlin classified philosophers as either “foxes” or “hedgehogs”, based on the classical Greek aphorism “The fox knows many things, but the hedgehog knows one great thing” [25]. Enzymes are commonly regarded as “hedgehogs”: each enzyme only knows how to catalyze one reaction. However, the enzymes of xenobiotic metabolism, such as P450 enzymes and GSTs, are undoubtedly “foxes” and able to catalyze the biotransformation of numerous substrates bearing diverse functional groups. As our understanding of these enzymes has increased, so has the range of known substrates and chemistries. Furthermore, the distinction that was once drawn between enzymes catalyzing xenobiotic metabolism and enzymes catalyzing metabolism of endogenous substrates has largely disappeared. Major chemical classes of GST substrates, such as quinones, epoxides, and hydroperoxides, encompass both exogenous and endogenous compounds. For example, acrolein (see Figure 1), a toxic α,β-unsaturated aldehyde that occurs in cooked foods and tobacco smoke [26–28] and is a metabolite of the cancer chemotherapeutic drug cyclophosphamide [29], is also formed endogenously, by the myeloperoxidase-catalyzed oxidation of threonine [30]; GSTs catalyze the detoxication of hydroperoxides (glutathione peroxidase activity), including both endogenous (e.g., hydrogen peroxide, lipid hydroperoxides) and exogenous (e.g., cumene hydroperoxide) compounds [31, 32].
Selected electrophilic substrates of GST enzymes. Top row, from left: acrolein; acrylamide; vinyl chloride (bioactivated to the reactive intermediates 2-chloroethylene oxide and chloroacetaldehyde); bottom row, from left: trans-stilbene oxide (TSO); 1-chloro-2,4-dinitrobenzene (CDNB); cumene hydroperoxide.
Overwhelmingly, the metabolic role of GSTs is to detoxify reactive electrophiles by catalyzing their reactions with glutathione, thereby reducing the likelihood of deleterious interactions between such reactive species and essential cellular components, especially proteins and nucleic acids. Many cancer chemotherapeutic agents are electrophilic compounds (or their metabolic precursors) and GST-catalyzed reactions are important pathways for the inactivation and elimination of many such drugs (e.g., 1, 3-bis(2-chloroethyl)-1-nitrosourea [33], cyclophosphamide, melphalan [34], etc.). Drugs from other therapeutic classes may be metabolized to electrophiles that are substrates for GST-catalyzed glutathione conjugation, for example, the phenylpropenal metabolite of the epilepsy drug felbamate [35]. Many other widely used drugs, including acetaminophen, clozapine, and furosemide, are metabolized to glutathione conjugates [36], although the reaction with glutathione is not necessarily dependent on GST catalysis in every case. Glutathione adducts are usually exported from the cell by the action of transporters such as the multidrug resistance protein MRP1 [37] and then processed into mercapturic acids (N-acetylcysteine conjugates) which are excreted in the urine [38] or bile [39]. Despite the important role of glutathione in detoxication, GST-catalyzed conjugations can also, in certain instances, lead to the generation of reactive intermediates. Dihaloalkanes are a notable case [40] and will be mentioned again, later in this article.
2.2. Human GSTs
Human GST enzymes include members of eight classes, assigned on the basis of sequence similarity: Alpha, Mu, Pi, Theta, Kappa, Zeta, Omega, and Sigma. Mammalian GSTs of the Alpha, Mu, and Pi classes bind with high affinity to matrices such as S-hexylglutathione-sepharose or S-hexylglutathione-agarose [41, 42], but GST enzymes from other classes, such as Theta, bind poorly or not at all [43]. (GST-GSH binding affinity is exploited in commercially available systems for expression of recombinant proteins as GST fusions, such as the pGEX vectors.) Because of their relatively high levels of expression and ease of purification, the Alpha, Mu, and Pi class GSTs have been studied more frequently than other classes of human GST enzymes.
The specificities of GST enzymes for the electrophilic substrate overlap considerably. For example, 1-chloro-2, 4-dinitrobenzene (CDNB), commonly used for spectrophotometric GST activity assays, is a substrate for most human GSTs (but not GST T1-1 [44] or GST T2-2 [45]). On the other hand, some substrates are relatively specific for particular GST enzymes, as discussed later.
Further discussion of the biochemistry of GSTs is presented in the monographs “Gluthione Transferases and Gamma-Glutamyl Transpeptidases” [46] and “Toxicology of Glutathione Transferases” [47], and in review articles [48–51].
3. GST Genes
3.1. Overview
Both animal and plant genomes encode large numbers of GST enzymes (and often, multiple pseudogenes with strong sequence similarity to GST genes), for example, 48 GST genes in the nematode Caenorhabditis elegans [52], 26 in the mosquito Aedes aegypti [53], and more than 70 in the Black Cottonwood poplar Populus trichocarpa [54]. The human genome encodes at least 18 expressed GST enzymes, in the eight sequence-similarity classes listed in Section 2.2 [5, 51, 55, 56]. Among these, the Alpha, Mu, Pi, and Theta classes have received most attention with respect to drug metabolism in humans. Some ambiguity persists in the enumeration of human GSTs. For example, the Alpha-class hGSTA5 gene product has been omitted from tabulations of human GSTs, because its expression has never been detected in human cells. However, the coding sequence is intact and an enzymatically active recombinant protein can be expressed; so it is likely that the enzyme is indeed expressed in humans, albeit under conditions that have not yet been discovered [57].
3.2. Human GST Gene Organization: Copy Number Variations
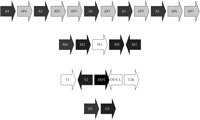
Human GST classes Alpha, Mu, Theta, and Omega all have multiple members, encoded by clusters of paralogous genes on a given chromosome (Figure 2). Deletion polymorphisms of the genes encoding human GST M1-1 and GST T1-1 are common in the human population [58]. A comprehensive review of the significance of these polymorphisms in pharmacology and toxicology was published in 2006 [59]. These deletions presumably arose by homologous recombination events. In both cases, although at least one of the neighbouring GST genes remains intact, individuals homozygous for the null allele show a clear phenotype with respect to glutathione conjugation of specific substrates. Homozygous deletion of the GSTM1 gene eliminates GST activity with respect to conjugation of the characteristic GST M1-1 substrate trans-stilbene oxide (TSO), as measured in lymphocyte homogenates [60–62]; indeed, the phenotypic polymorphism was discovered (with the substrate trans-4-phenyl-3-buten-2-one) before the gene was cloned [63, 64]. Similarly, homozygous deletion of the GSTT1 gene eliminates GST activity with respect to conjugation of the characteristic GST T1-1 substrates methyl bromide (CH3Br) and dichloromethane (CH2Cl2), as measured in erythrocyte homogenates [65, 66]. These biochemical phenotypes have toxicological correlates. For example, genotoxicity of TSO in cultured human lymphocytes (as measured by induction of sister chromatid exchanges) is significantly elevated in GSTM1-null individuals [67], and, as discussed later, clinical toxicity of methyl bromide may be strongly determined by GSTT1 genotype.
Organization of selected human GST gene clusters. “P” indicates a pseudogene (e.g., AP3). The GST classes shown are Alpha (chromosome 6p12) [68, 69], Mu (chromosome 1p13) [45, 46], Theta (chromosome 22q11.2) [70, 71], and Omega (chromosome 10q24.3) [72, 73]. (A reverse transcribed pseudogene of the Omega class, found on chromosome 3 [72], is not shown.) GST genes are shown as white text on black background and pseudogenes are shown as black text on a grey background. The direction of each gene is indicated by the arrow. The genes GSTM1, GSTT2B, and GSTT1, each of which is commonly deleted, are shaded white. Genes DDT and DDT-L, in the Theta cluster, encode the enzyme D-dopachrome tautomerase. DDT-L is commonly deleted along with GSTT2B. The figure is not drawn to scale.
Deletion polymorphisms are a specific case of copy-number variation, and the recombination mechanisms that cause deletions can also cause duplications. The extent and significance of gene copy number variation in the human genome has only recently started to become clear, lagging behind the cataloguing of several million human single nucleotide polymorphisms (SNPs) [74]. Human P450 2D6, for instance, provides an important example of copy-number variation—both deletions and duplications—affecting the disposition of drugs, such as the tricyclic antidepressants and tamoxifen [75–77]. The GSTM1 gene is duplicated in some individuals, conferring a +/++ genotype and “ultrarapid” metabolism of TSO [78, 79].
3.3. Molecular Epidemiology of GST Deletion Polymorphisms
The relationships between GST polymorphisms and disease risk have been examined in a vast number of molecular epidemiological studies, beginning around 1990. While these studies are not pharmacogenetic per se, they may be relevant to the more general question of whether human GST polymorphisms influence responses to xenobiotic compounds, since environmental exposures contribute to the risk of specific cancers and other prevalent diseases. A PubMed search using the search string “GST cancer risk” retrieved 599 references (Jan. 2010), and many noncancer diseases have also been studied, for example, Parkinson's disease, Alzheimer's disease, asthma, coronary artery disease, and rheumatoid arthritis (reviewed in [59]). The HuGE Navigator “searchable knowledge base of genetic associations and human genome epidemiology” (http://www.hugenavigator.net/) retrieved 1,027 publications in response to a search for gene symbol GSTT1. Almost all of the molecular epidemiological studies of GSTs have tested the effects of some or all of three genotypes: GSTM1-null, GSTT1-null, and the GSTP1 single-nucleotide polymorphism that results in the coding sequence change Ile105Val [80, 81].
Several circumstances account for the fact that GST polymorphisms have been subject to so many epidemiological analyses. First, the polymorphisms are easily tested. For example, routine PCR analysis can classify individuals as GSTT1 homozygous null versus GSTT1-present and GSTM1 homozygous null versus GSTM1-present; see, for example, [82]. (The limitations of such analysis are discussed later.) Second, homozygous null individuals are common, contributing statistical power to molecular epidemiological analyses. The prevalence of GSTM1 homozygous null individuals in “Caucasian” and Asian populations is about 50%, with a substantially lower frequency among Africans and African Americans. For the GSTT1 homozygous null genotype, the corresponding figures are about 20% for “Caucasian” and African-American populations, but about 50% for Asians [59]. Third, as mentioned earlier, the polymorphisms give rise to detectable phenotypes, in terms of metabolism of some specific substrates. Finally, the acknowledged importance of GSTs in the disposition of toxic compounds and in defense against oxidative stress [83] provides a prima facie justification for testing associations with the risk of cancers and degenerative diseases.
Bolt and Thier have provided an extensive review of the GST molecular epidemiology literature up to about 2005 [59]. Since that time, several new meta-analyses have been published under the auspices of the “HuGE” Human Genome Epidemiology Network, formed in 1998, which facilitates the preparation of “systematic, structured, peer-reviewed synopses of epidemiologic aspects of human genes in relation to specific diseases” [84]. A summary of these meta-analyses is given in Table 1. Overall, it can be seen that GSTT1 or GSTM1 null genotype confers at most a small (less than 50%) increased risk of certain cancers, while many other results are negative (no statistically significant increased risk).
HuGE reviews of GST polymorphisms and disease risk.
The great majority of the molecular epidemiological investigations published to date suffer from a serious limitation: genotypes
were assessed by “yes-no” PCR methods that do not measure gene copy number and therefore cannot distinguish between heterozygous
(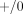 ) and homozygous (
) and homozygous (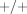 or even
or even 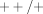 ) non-null genotypes. As noted by Minelli et al. [94], “classifying the genotype [only] as “present” or “null” implies a recessive model (one or two copies versus absence of
the risk allele), which may not reflect the true underlying genetic model and thus may not provide a valid and accurate estimate
of the genetic risk. GSTT1 or GSTM1 copy number variations are correlated with altered enzyme activity, and analysis in a dose-dependent manner would best describe
any disease outcome association.” Analytical methods that assess copy number, such as real-time PCR [95–97], are now available; older and less informative genotyping methods should be abandoned [98].
) non-null genotypes. As noted by Minelli et al. [94], “classifying the genotype [only] as “present” or “null” implies a recessive model (one or two copies versus absence of
the risk allele), which may not reflect the true underlying genetic model and thus may not provide a valid and accurate estimate
of the genetic risk. GSTT1 or GSTM1 copy number variations are correlated with altered enzyme activity, and analysis in a dose-dependent manner would best describe
any disease outcome association.” Analytical methods that assess copy number, such as real-time PCR [95–97], are now available; older and less informative genotyping methods should be abandoned [98].
Several other biases and weaknesses are often found in molecular epidemiological investigations and tend to reduce confidence in published results. (i) Post hoc analysis: data can be recategorized so as to increase statistical significance, by constructing a new hypothesis after the data have already been acquired. For example, in a study of the GSTM1 null polymorphism and ovarian cancer risk [99], very high statistical significance was reported for elevated risk of GSTM1 null genotype with respect to the incidence of combined clear cell and endometrioid pathological subtypes of ovarian cancer, but this combination of subtypes was constructed post hoc, and the biological rationale explaining why these subtypes in particular should be affected by GSTM1 status is not compelling. (ii) Publication bias: studies which find significant associations are more likely to be published than studies which find no association. The recent HuGE meta-analysis of asthma studies [94] noted “clear absence of small studies with negative results, suggesting the presence of publication bias.” (iii) Small sample size: published studies of the GSTT1 and GSTM1 polymorphisms include samples as small as 34 patients with chronic obstructive pulmonary disease [100], 51 liver transplant recipients [101], and 43 schizophrenia patients [102], and such small samples are unlikely to yield reliable data. (iv) Lack of clear biological rationale: is it reasonable to expect that the presence of a non-null GSTT1 gene is associated with, for example, significantly better response to the surgical correction of an enlarged scrotal vein [103]? In contrast, an epidemiological association is more credible when a plausible connection exists between a presumed causative agent and the disease outcome. A strong association between GSTM1 null genotype and hepatocellular carcinoma has been reported in studies in Guangxi, China [104, 105]. The very high incidence of liver cancer in this region is attributed, at least in part, to prevalent aflatoxin contamination of grain, and GST M1-1 catalyzes the detoxication of aflatoxin epoxide [106].
4. Human GST Theta Genes and Enzymes
4.1. Characteristics of Theta Class GSTs
Theta class GSTs are distinct from the Alpha, Mu, and Pi (A-M-P) class enzymes in many respects, including sequence, catalytic activity, and structure [107]. GST Theta genes are evolutionarily very distant from the A-M-P genes and probably diverged from the ancestral A-M-P gene long before the divergence of plants and animals; Theta class GSTs are found in plants, but A-M-P GSTs are absent [108]. The human genome encodes two Theta class GSTs, GST T1-1 and GST T2-2 [70], and possibly a third form, GST T2B-2B (see below). Three Theta class GSTs are encoded on the mouse genome [109].
As noted earlier, Theta class enzymes are distinct from the A-M-P enzymes in failing to bind tightly to glutathione affinity matrices and having little or no activity with the standard GST substrate CDNB [110]. Theta class GSTs have a distinctive (although not unique [111]) activity: catalysis of the conjugation of halo- and dihaloalkanes with glutathione [40], a reaction which can result in the formation of reactive intermediates related to the “sulfur mustards” (S-haloalkanes) [112]. Therefore, Theta class GSTs can catalyze bioactivation [113] as well as detoxication processes, as discussed further below. An intriguing case report [114] suggests that GST T1-1-dependent activation of haloalkanes can be clinically important. Two workers were exposed to a very large inhaled dose of methyl bromide when they entered a sealed mill building being fumigated with the gas, and failed to wear self-contained breathing apparatus. Neurotoxic effects were very severe in one worker but mild in the other. Laboratory investigation showed that the severely affected individual had GST (presumably GST T1-1) activity towards methyl bromide, while the mildly affected individual (presumably a GSTT1 homozygous null) did not. This result, while representing little more than an anecdotal report, is consistent with a determinative role for GST T1-1 in methyl bromide toxicity.
The active sites of Theta class GSTs are also distinctive. In A-M-P class GSTs, a conserved tyrosine residue near the N-terminus forms a hydrogen bond to the thiol sulfur atom of glutathione in the G-site; in Theta class GSTs, a serine residue occupies the corresponding position [48, 115–117]. The G-site of human Theta class GSTs is deeply buried and covered by the C-terminal “tail”, approximately 40 amino acid residues that form a helix-loop-helix extension [116]; this tail also makes the H-site of Theta class enzymes relatively inaccessible [118]. The small size of the H-site is consistent with the selectivity of Theta class GSTs for small xenobiotic substrates, such as dichloromethane [119]. This preference for small xenobiotic substrates is reminiscent of the behaviour of cytochrome P450 2E1, an enzyme which is also notable for an unusually small active site [120], and which catalyzes the oxidation of small substrates such as dimethylnitrosamine [121] and ethanol [122]. Indeed, there may be cases where toxicants are activated by P450 2E1 to reactive species that are subsequently detoxified by GST T1-1-catalyzed glutathione conjugation. Possible examples include vinyl chloride (a plastics monomer and industrial carcinogen) [123, 124], acrylamide [125], and benzene [126, 127].
4.2. Chromosomal Organization of the GSTT Genes
The structure of the human GST Theta region [70, 71] is remarkable (Figure 3). The GST1 and GST2 are oriented head-to-head. GSTT2B was originally referred to as a probable pseudogene, GSTT2P [70], but is now annotated as “glutathione S-transferase theta 2B (gene/pseudogene)” on the NCBI genome database. (Pseudogenes identified as GSTTP1 and GSTTP2 are also annotated on the database but have not been studied in detail.) The GSTT2, DDT, DDT-L, and GSTT2B genes are found within a 61 kb inverted repeat sequence which was recently discovered to be the site of a prevalent (allele frequency approximately 50%) deletion polymorphism that spans the entire GSTT2B gene [71]. Additional characteristics of this previously unknown deletion polymorphism may have important implications for pharmacogenetic studies of the GST Theta genes. Surprisingly, deletion of the GSTT2B gene appears to result in greatly reduced expression of the GSTT2 gene, by an as-yet unknown mechanism; the GSTT2B deletion shows linkage disequilibrium with the much-studied GSTT1 deletion polymorphism, with a very low frequency of alleles carrying deletions of both GSTT1 and GSTT2B [71]. Furthermore, the extent of linkage disequilibrium was very different among three population samples examined: very strong in a northern/western European ancestry sample, strong in a Japanese-Chinese sample, but absent in a Nigerian Yoruba sample [71]. Further investigation of the molecular genetics of human GSTs is very much needed.
4.3. D-Dopachrome Tautomerase
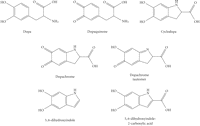
Another intriguing feature of the GST Theta gene region is the presence within the 61 kb inverted repeat of the overlapping, head-to-head DDT (D-dopachrome tautomerase) and DDT-L (D-dopachrome tautomerase-like) coding sequences. As Coggan et al. observed, the proximity of the DDT and GSTT2 genes is “if nothing else, an interesting coincidence.” Does the proximity of these genes have any functional significance? First, we should consider the biochemistry of the DDT gene product, D-dopachrome tautomerase. L-Dopachrome is a tyrosine metabolite required for biosynthesis of the skin pigment melanin [128]. Hydroxylation of tyrosine forms dopa (dihydroxyphenylalanine), which is oxidized to the quinone, dopaquinone (Figure 4); both oxidations are catalyzed by tyrosinase [129]. Dopaquinone can cyclize to give cyclodopa, which undergoes further oxidation to yield dopachrome [130]. In the pathway to eumelanin, L-dopachrome tautomerase (also known as L-dopachrome isomerase) catalyses the isomerization of dopachrome, via a tautomeric form (dopachrome tautomer), to 5,6-dihydroxyindole-2-carboxylic acid [131]. (Alternatively, dopaquinone can react with cysteine to form cysteinyldopa, leading to synthesis of phaeomelanin [130].) All of these metabolites are derived from the natural “L” enantiomer of the amino acid tyrosine. In the course of studies on L-dopachrome tautomerase, researchers used the nonnatural “D” enantiomer of dopachrome as a control; to their surprise, they found that D-dopachrome also underwent tautomerization. Liver (rather than melanin-producing cells) was found to have high D-dopachrome tautomerase activity [132]. D-Dopachrome tautomerase (also known as D-dopachrome decarboxylase) catalyzes the decarboxylation of D-dopachrome to give 5,6-dihydroxyindole [133]. The cytokine macrophage migration inhibitory factor (MIF) [134], another member of the tautomerase superfamily [135], catalyzes the conversion of D-dopachrome into 5,6-dihydroxyindole-2-carboxylic acid [136]. Since D-dopachrome is not found in cells, these enzymes presumably have different endogenous substrates, possibly including phenylpyruvate [137]. The physiological role of D-dopachrome tautomerase remains enigmatic.
There are at least some hints of a functional relationship between D-dopachrome tautomerase and GSTs. (i) Dopachrome and related quinones are substrates for GST-catalyzed glutathione conjugation [138, 139]. (ii) The DDT and GSTT2 genes may be coordinately regulated; a recent genomewide analysis identified both genes among a small set of genes that are expressed differentially in a comparison between strains of spontaneously hypertensive rats and the control (Wistar-Kyoto) strain [140]. (iii) A recent proteomic analysis identified D-dopachrome tautomerase as being strongly (more than tenfold) induced in rat liver following exposure to the hepatotoxicant, carbon tetrachloride (CCl4) [141], and GST enzymes may protect against CCl4-induced hepatotoxicity [142]. Further research is needed to clarify the biological role of D-dopachrome tautomerase and its possible interactions with the glutathione/GST system.
4.4. Bioactivation of Mutagens by Theta Class GSTs
As mentioned earlier, GST-catalyzed conjugation of dihaloalkanes gives rise to toxic reactive intermediates. Ethylene dibromide (EDB), a compound that has been used as an antiknock additive in gasoline and as an insecticide, can cause fatal liver, kidney, and cardiac toxicity [143]. EDB is probably the best-characterized example of GST-catalyzed bioactivation [144, 145]. EDB-glutathione conjugation catalyzed by GST T1-1 leads to generation of an electrophilic sulphonium ion that can form covalent adducts with macromolecular targets [146] (Figure 5). It would be very informative to test whether GSTT1 genotype affects the outcome of EDB poisonings in exposed individuals (such as accidental poisonings or suicide attempts).
Mammalian enzymes catalyzing the metabolic activation of xenobiotics can be expressed in bacterial strains for the detection of mutagens [147], as has been demonstrated for aromatic amine N-acetyltransferases [148], P450 enzymes [149], and sulfotransferases [150]. Thier and colleagues demonstrated that rat GST T1-1 (previously known as form 5-5) [151] and human GST T1-1 [152] expressed in Salmonella typhimurium strains catalyze the bioactivation of dihaloalkanes to mutagens that can readily be detected by the Ames test (reversion mutation assay).
4.5. Coding-Sequence SNPs Affecting GST Theta Proteins
With the exception of the GSTP1 SNP mentioned earlier, there have been relatively few studies of coding-sequence SNPs affecting GST proteins. However, the Environmental Genome Project [153] and several recent publications [154–161] have uncovered many new variants in the Mu, Pi, Theta, and Omega GST classes. Characterization of the functional consequences of these variants is required for a thorough understanding of the pharmacogenetics of GSTs [162] and can also provide insight into the structure-activity relationships of the enzyme proteins. The effects of coding-sequence SNPs affecting the human Theta class GSTs are under investigation [163, 164]. A GST expression system based on Escherichia coli strains bearing a lacZ reversion target has been constructed and is being applied in studies of the functional consequences of nonsynonymous SNPs in human GSTT1 [164]. Reported nonsynonymous SNPs in this gene (Entrez SNP database at NCBI; Environmental Genome Project database at www.genome.utah.edu/genesnps/; [160]) encode the protein variants A21T, L30P, D43N, F45C, T65M, R76S, T104P, D141N, V169I, and E173K. In our first study, we expressed the D141N and E173K variants. The D141N variant behaved similarly to the wild-type enzyme, in terms of expression level and specific activities towards a variety of xenobiotic substrates. However, the variant displayed a very much reduced activity for the activation of EDB to a mutagen. Variant E173K, in contrast, was poorly expressed and inactive with most substrates, and the protein appears to be improperly folded, as judged by its altered thermal denaturation profile. Extension of these studies to additional GSTT1 SNPs is in progress.
5. Closing Remarks
A large number of epidemiological studies have tested possible associations between GST polymorphisms, such as the GSTM1 and GSTT1 deletions, with disease risk or therapy outcome. Some meta-analyses have indicated statistically significant but small increases in risk for specific genotypes, while many studies have been negative. However, the genetic analysis used in most of these studies has been limited, especially by the failure to determine between heterozygous and homozygous genotypes (gene dose). GST activity is highly variable among individuals, but genetic factors may account for only a fraction of this variability. Although clear cases of clinically relevant pharmacogenetic consequences and toxicogenetic GST polymorphisms remain very few, greater understanding of the numerous factors affecting GST expression and activity, accompanied by more incisive genetic analysis, may reveal further connections between GST genotypes and individual responses to drugs and toxic compounds.
Acknowledgments
This work was supported by a grant from the Natural Sciences and Engineering Research Council of Canada. The author wishes to thank Professor Cortland Griswold, University of Guelph, for helpful discussions, and Professor Bengt Mannervik, Uppsala University, for providing valuable comments on the manuscript draft.
Abbreviations
- A-M-P:
- Alpha, Mu, and Pi classes of GSTs
- CDNB:
- 1-chloro-2,4-dinitrobenzene
- GST:
- glutathione transferase
- TSO:
- trans-stilbene oxide.
- Received February 2, 2010.
- Accepted March 22, 2010.
- © 2010 P. David Josephy.