Accessory Proteins in the Biogenesis of G Protein-Coupled Receptors
- QYZ. E-mail qzhou{at}uci.edu; fax 949-824-4855.
Abstract
Intracellular accessory proteins can be critical for G protein–coupled receptor (GPCR) biogenesis, including aspects of GPCR trafficking. Recent discoveries include the identification of multiple membrane-associated proteins that dictate not only the intracellular sequestration and/or transport of GPCRs, but also modulate—quite dramatically—GPCR ligand specificity subsequent to delivery to the cell surface. These exciting discoveries have shifted earlier paradigms of GPCR functionality.
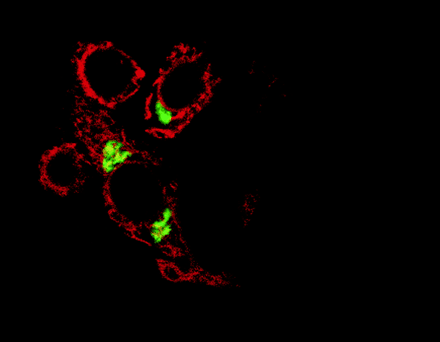
Proteins that reside in the endoplasmic reticulum, such as the dopamine receptor-associated DRiP78 protein (shown in red)
are being increasingly discovered as essential for the transport of G protein-coupled receptors through the Golgi apparatus
(green) and to their destinations at the cell surface. [Reprinted with permission from Nature Cell Biology.]
INTRODUCTION
As the single largest family of cell surface receptors, G protein–coupled receptors (GPCRs) recognize a vast diversity of cellular modulators, including hormones, neurotransmitters, nucleotides, peptides, ions, and photons (1). GPCRs thus control a number of intracellular biochemical changes, from enzyme and ion channel activation to vesicular transport and gene expression, and thus they are pivotal as drug discovery targets. All GPCRs share the common feature of possessing seven transmembrane domains, and traditionally, they have been perceived as monomeric proteins that interact with heterotrimeric G proteins at the interior surface of the cell membrane so as to mediate signal transduction. In recognition of a wider range of protein–protein interactions in which GPCRs participate, however, the monomeric model of GPCRs has been expanded in regard to receptor signaling as well as to receptor biogenesis (2). In this review, we outline some of the evidence that points to the requirement of membrane-associated accessory proteins in GPCR biogenesis. The dependence of GCPRs upon such proteins has implications not only for our understanding of cell regulation, but also for the development of novel therapeutics.
VISUAL AND OLFACTORY PROTEINS
The importance of membrane-associated accessory proteins for the biogenesis of GPCRs was first established from studies of the rhodopsin and odorant receptors. The Drosophila cyclophilin gene ninaA (neither inactivation nor afterpotential A) and its mammalian homolog, the RanBP2-encoding gene (RanBP2 for Ran binding protein 2), both of which are selectively expressed in photoreceptor cells, have been shown essential for the efficient localization of opsins at the cell surface (3,4). Both genes encode cyclophilins, a widely conserved family of peptides that are targeted by cyclosporin A (CsA), a powerful immunosuppressant. Although the in vivo functions of the cyclophilins are poorly understood, they display peptidyl prolyl isomerase (PPIase) activity in vitro (5,6). Consequently, original hypotheses pitched NinaA as a catalyst for rhodopsin folding (7,8,9). Although the PPIase activity of NinaA (and other cyclophilins) has not been verified in vivo, it has become apparent that NinaA forms a stable complex with R1-6 rhodopsin in vivo (10) and functions as a chaperone, promoting the trafficking of rhodopsin out of the endoplasmic reticulum (ER).
Similarly, RanBP2 promotes the delivery of red/green (R/G) opsin to the cell surface. Unlike NinaA, RanBP2 does not appear to possess transmembrane domains, but does contain a prenylation motif for membrane anchoring at its C terminus (11). In addition to its homology with cyclophilin, RanBP2 contains a domain that binds to the nuclear GTPase Ran (i.e., Ran-binding domain 4; RBD4) and that is not present in NinaA. The association of RanBP2 with R/G opsin occurs via the RBD4 domain, and is stabilized by the cyclophilin domain (4). Ran proteins are abundant nuclear GTPases putatively involved in nuclear mRNA trafficking. The role of Ran-binding as an element of the chaperoning of rhodopsin is not at all established and may be an unrelated feature.
More recently, in C. Elegans, analysis of the odr-4 mutation (for abnormal odorant response 4) has demonstrated that a protein of 445 amino acids is necessary for the efficient targeting of odorant receptors to olfactory cilia (12). ODR-4 has a structural topography similar to that of NinaA, although there is no obvious sequence similarity between the two proteins. ODR-4 appears localized to intracellular membrane compartments such as the ER, the Golgi apparatus, and transport vesicles, and may promote receptor biogenesis as a chaperone, although direct physical interaction with the odorant receptor has not yet been demonstrated. It is also possible that ODR-4 may affect general vesicular transport between intracellular membrane compartments. However, the role of ODR-4 in the cell surface expression of odorant receptors is selective; in its absence, a number of odorant receptors are still normally targeted, suggesting that other localization systems are present as well (12).
GPCR OLIGOMERIZATION
In the past few years, numerous examples have surfaced in which GPCR functionality depends upon dimeric associations of seven-transemembrane polypeptides and even multimeric complexes including seven-transmembrane polypeptides (13,14). In some cases—most notably, the opioid receptor subtypes—alternative quartenary structures involving GPCRs display distinct pharmacological characteristics as compared to the monomeric polypeptides (15). A distinct role for GPCR heterodimerization in the transport of newly synthesized receptors to the cell surface has been shown most clearly with the GABAB receptor.
Despite ligand availability, and the capacity of GABA (γ-aminobutyricacid) to stimulate high-affinity GTPase activity in brain membranes, the identity of the GABAB receptor remained elusive for many years. Eventually, a high-affinity radiolabelled antagonist allowed the identification and cloning of a seven-transmembrane polypeptide bearing sequence similarity to the metabotropic glutamate, or type C (class III), GPCRs (16). The cloned GABAB receptor 1 (GABABR1) exhibited many properties of the endogenous GABAB receptor, in terms of tissue distribution and antagonist binding, but it bound agonist poorly in recombinant systems. Transfection of the gene for GABABR1 into mammalian cells resulted primarily in immature glycoprotein that was restricted to intracellular membranes (17). Subsequently, several research teams identified a second receptor, GABABR2, which, when coexpressed with GABABR1, reproduced the pharmacology of endogenous GABAB receptor activity (17–,21). GABABR2 is also a class III GPCR, sharing 35% sequence identity with GABABR1. Physical interaction between the homologs was evidenced in these studies by colocalization, two-hybrid screening, and coimmunoprecipitation.
GABAB receptor heterodimerization is mediated by a coiled-coil domain interaction within the C termini of the two polypeptides (17). Analogously, the p24 proteins, which contain a single transmembrane domain and are important mediators of vesicular transport between intracellular compartments, also undergo C-terminal coiled-coil interactions (22). Thus far, we can only speculate that GABAB receptor heterodimerization may promote the recruitment of other cellular factors central to cargo export from the ER. Regardless, an interesting possibility exists that GABAB receptor heterodimerization provides quality control to ensure proper receptor maturation prior to ER export. In this vein, GABABR2 appears not to be directly involved in recognition of GABA, implying that its association with GABABR1 serves strictly a chaperone-like function. Furthermore, a basic RXR(R) motif has recently been identified within the GABABR1 C terminus and mediates ER retention (23). Conceivably, the interaction of GABABR1 with GABABR2 masks the ER retention motif and allows for the concerted delivery of functional receptor to the plasma membrane. Whether this process can be exploited for cellular control of receptor expression is intriguingly left open to future exploration.
RECEPTOR ACTIVITY–MODIFYING PROTEINS
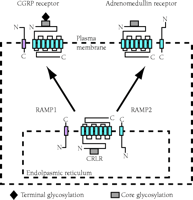
The discovery that a GPCR activity is regulated through its association with novel group of proteins (i.e., the receptor activity–modifying proteins; RAMPs) has provided a dramatic shift in our understanding of the molecular basis of GPCR pharmacology. In attempts to expression clone the receptor for the calcitonin gene-related peptide (CGRP), a cDNA that encoded a 148-residue type I transmembrane protein was isolated by monitoring cellular responsiveness to CGRP (as assayed by means of intracellular cAMP production) (24). The novel protein was named receptor activity–modifying protein 1 (RAMP1), and the products of two homologous cDNAs were termed RAMP2 and RAMP3. Rather than functioning as a receptor for CGRP, however, RAMP1 activated the endogenous calcitonin receptor-like receptor (CRLR) that had previously been regarded as unrelated to cellular responsiveness to CGRP. Whereas neither CRLR nor RAMP1 alone could endow transfected mammalian cell types, including COS-7, HEK-293, and Swiss3T3, with the ability to bind or respond to CGRP, the coexpression of CRLR along with RAMP1 resulted in cells that both bound and responded to CGRP (25). Impressively, cotransfection of RAMP2 or RAMP3 with CRLR resulted in cells that preferentially responded not to CGRP, but to adrenomedullin, another member of the calcitonin family of peptides (Figure 1⇓) (25,26).
The modification of GCPR ligand specificity by accessory proteins. Receptor activity–modifying proteins (RAMPs) are crucial not only to the delivery of calcitonin receptor–like receptor (CRLR), a seven-transmembrane GCPR, to the plasma membrane, but also determine the specificity of CRLR for ligand. Association of CRLR with RAMP1 results in a receptor for calcitonin gene–related peptide (CGRP), whereas association of CRLR with RAMP2 results in a receptor for adrenomedullin. [Image courtesy of Nature (www.nature.com). (See reference (25).]
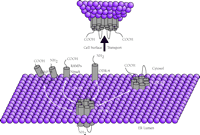
GPCR association with transmembrane accessory proteins in the ER. A summary of the GPCR–protein interactions that are discussed in the text. 1) The GABABR1 receptor associates with homologous GABABR2 in forming the heterodimer (shown exiting the ER) required for vesicular transport to the cell surface. 2) An odorant GPCR has been shown to associate with ODR-4, an essential type II membrane-anchored protein. 3) Receptors CRLR and Drosophila rhodopsin require association with type I membrane proteins, such as RAMPs and NinaA, respectively. 4) The dopamine D1 receptor binds to DRiP78 (possessing two internal transmembrane domains) in coordinating ER export.
The RAMPs are required for CRLR transport to the cell surface, raising translocation from 3% to approximately 25%. Conversely, RAMP expression is limited to intracellular membranes except in the presence of its cognate GPCR, with which RAMPs associate at the cell surface (25,27,28). Thus, RAMP–GPCR association may play two important roles: receptor transport followed by receptor functionality. In fact, radioligand and cross-linking studies show continued RAMP–GPCR association following agonist binding (27,28), with clear implications for the development of novel antagonists that could disrupte the GPCR-RAMP interaction, rather than strictly interfer with the GPCR–agonist association.
Cell responsiveness to yet another member of the calcitonin family of peptides, namely, amylin, also depends on the RAMPs, in this instance working in concert with expression of the calcitonin receptor (CTR) (27,29). However, it appears that the specific type of RAMP required for amylin receptor activity also depends upon the cellular environment (i.e., COS-7 versus CHO), suggesting that a number of other cellular components may be involved in the association and/or transport of the CTR. CTR and CRLR share significant homology to group B GPCRs that recognize peptide hormones, such as parathyroid hormone (PTH), secretin, and vasoactive intestinal polypeptide (30). The role of the RAMPs in the pharmacology of these receptors has not yet been established. In addition, much more remains to be determined regarding the mechanism by which RAMPs so dramatically facilitate GPCR transport, and whether they are directly or allosterically involved in ligand binding.
DRiP78 AND THE DOPAMINE D1 RECEPTOR
Another ER membrane–associated protein, DRiP78, was recently implicated in the trafficking of the D1 receptor (31). DRiP78 (for dopamine receptor interacting protein 78) appears to contain two centrally located transmembrane domains, with cytosolic orientation of both the N and C termini. The C terminus contains a zinc finger domain and associates with a transport motif, FxxxFxxxF, within the proximal C terminus of the D1 receptor. This Dl receptor transport motif is highly conserved among GPCRs and can control the ER export properties of recombinant proteins to which it is fused. The motif is highly sensitive to mutation at hydrophobic residues; substitution of any of the conserved phenylalanine residues with alanine results in arrest of D1 protein within the ER. Furthermore, truncations and/or substitutions within this domain have resulted in the trapping of several GPCRs, notably luteinizing hormone (LH/CG) and vasopressin (V2) receptors, in the ER (32,33). The direct association of DRiP78 with the FxxxFxxxF motif of the D1 receptor, required for efficient receptor export from the ER, was determined by the expression of competing peptides (31). Moreover, D1 receptor transport was intimately sensitive to the cellular levels of DRiP78 available for association. Interference with or augmentation of these levels significantly slowed receptor biogenesis, suggesting that DRiP78 may play a dual role in D1 receptor transport; specifically, DRiP78 appears to impede D1 export from the ER until presentation of its C terminus to some, as yet undetermined, transport complex can take place. The conservation of the DRiP78 binding motif within the C terminus of many GPCRs suggests that DRiP78 may play a broader role in GPCR transport. In fact, initial studies indicate that muscarinic receptor expression is also influenced by this accessory protein.
CONCLUSIONS
The studies outlined above all characterize the export of GPCRs from the ER as a highly coordinated process. Because GPCRs are typically expressed in very low cellular quantities, the requirement for accessory protein-based transport may serve an important function in quality control, ensuring the strict and efficient expression of functional receptors at the cell surface. The composite discovery of chaperones, dimerization of GPCRs, and accessory proteins that facilitate GPCR biogenesis raises intriguing questions. To what extent do these protein–protein interactions direct receptor folding, receptor trafficking, and vesicular budding? Are unifying transport mechanisms involved in such seemingly diverse associations? More intriguing is the question of whether cells possess regulation over the sequestration of GPCRs in the ER. Regardless of the mechanisms involved, pharmacologists are presented now with novel targets for the development of therapeutics interfering with or modulating critical GPCR–accessory protein interactions (Figure 2⇑).
Acknowledgments
This work was supported by grants from the NIH (MH57889), the Alfred E. Sloan Foundation, and the Optical Biology Shared Resource Grant (CA-62203).
Footnotes
-
Qun-Yong Zhou, Ph.D., is Assistant Professor of Pharmacology at the University of Calfornia at Irvine. Jason C. Bermak is a graduate student in his laboratory.
- © American Society for Pharmacology and Experimental Theraputics 2001