The Path Well Traveled: Using Mammalian Retroviruses to Guide Research on XMRV
Since their discovery thirty years ago (1), human retroviruses have become a major worldwide health concern. Human immunodeficiency virus type 1 (HIV-1), which infects approximately thirty-three million individuals, is the causative agent of AIDS and is also associated with neoplasia and neurologic disease. Another 10–20 million people are infected with a different retrovirus, human T cell leukemia virus type 1 (HTLV-1), which is the cause of adult T-cell leukemia/lymphoma (ATL) and several inflammatory disorders, including a progressive neurological disorder (HTLV-1-associated myelopathy/tropical spastic paraparesis; HAM/TSP) (2).
Recently, a previously unknown retrovirus, xenotropic murine leukemia virus-related virus (XMRV), was detected in human tissue (3). XMRV has now been linked to two distinct types of human disease and infectious virus has been isolated from the blood of infected individuals (3, 4). Although this virus is only beginning to shed its secrets, the large body of knowledge generated during decades of studying both human and non-human retroviruses has provided important clues about its biology and approaches for the development of therapeutic regimens.
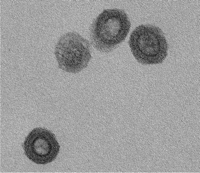
XMRV was initially discovered by screening tissue from prostate cancer patients for the presence of viral sequences (3). Retroviral DNA was isolated from several of the samples. The sequences from this DNA are only distantly related to HIV-1 and HTLV-1 but are very closely related to a type of retrovirus called xenotropic murine leukemia virus (X-MLV) (5) (Box 1). Exogenous expression of these sequences in cells further supported the notion that this novel human retrovirus is related to a murine retrovirus: viral particles were generated that were both morphologically similar to murine leukemia viruses (MLVs) (Figure 1) and were composed of proteins that were recognized by antibodies to the MLVs (4, 6, 7). The association between XMRV and prostate cancer was strengthened by a recent report of the presence of XMRV proteins, as well as nucleic acid sequences, in prostate cancer tissue (7). This group observed expression of XMRV proteins in the malignant cells in these samples, in contrast to the earlier report that XMRV was present in nonmalignant (stromal) cells adjacent to the malignancy (4, 7). Moreover, evidence of XMRV infection was found in over a quarter of samples analyzed, and XMRV was found at a higher frequency in more aggressive cancers than in less aggressive ones.
Thin-section electron microscopy transmission of XMRV produced from human cells. Bar denotes 100 nanometers.
Xenotropism, or the Ability to Infect Other Species
As part of its life cycle, a retrovirus inserts a DNA copy of its genome into a chromosome in the host cell. When this occurs in a germ cell, the integrated viral DNA can be passed to future generations along with the host genome and is then referred to as an endogenous retrovirus (ERV). Laboratory mouse strains carry ERVs of several different types of mouse leukemia virus (MLV), including xenotropic MLVs (X-MLVs). X-MLVs are not able to infect cells derived from laboratory mice (or the mice themselves), but they can infect cell lines from a number of other species, including human. This is because the receptor for X-MLV, which is functional in humans and most other mammals, is mutated in laboratory mouse strains. Because X-MLV can infect human cells, it is not surprising that it would be closely related to the human retrovirus XMRV.
Data from Lombardi et al. (4), associating XMRV with a different type of disease, chronic fatigue syndrome (CFS), raises additional concerns for the public health. The authors report that XMRV was present in a high percentage (67%) of individuals with CFS. Evidence of XMRV infection was detected in leukocytes isolated from peripheral blood; further studies revealed that both T and B lymphocytes could be infected with XMRV. Importantly, this study also demonstrated that infectious virus was present in infected individuals: XMRV could be transmitted from both activated lymphocytes and plasma from infected individuals. These individuals also had antibodies specific for this type of retrovirus, showing that XMRV can elicit an immune response.
Despite associations with both prostate cancer and CFS, many key questions remain regarding the pathogenicity, prevalence and replication of XMRV in the human population. From the few studies published to date, it is not yet clear whether XMRV is a direct cause of one or both of these diseases, indirectly contributes to their development, or is a benign virus that is either expressed at higher rates in those patient populations or is more common in certain cohorts. In contrast to what was observed in studies of samples from prostate cancer patients in the US, two independent groups in Germany have reported observing little (1/150) (8) or no (0/589) (9) evidence of XMRV in prostate tumor samples. These differences could reflect geographical differences in the distribution of XMRV or differences in the assays used to detect infection. In the CFS study, the samples analyzed were primarily from individuals in areas of previous CFS “outbreaks,” which may have overrepresented the proportion of these patients with a viral etiology. Better understanding of the disease association and prevalence of this virus will require the generation of standard reagents, such as monoclonal antibodies generated against XMRV proteins, the development of sensitive and specific well-validated XMRV detection assays, and subsequent large scale clinical studies.
Other important questions that have not yet been addressed involve the mode of transmission, the level of replication, and the origin of XMRV in the human population. Although it is tempting to speculate that, like HIV-1 and HTLV-1, XMRV is spread by sexual contact, via blood and blood products, or from mother to child, no published studies have addressed whether this is indeed the case. Although HIV-1 replicates at high levels in the blood of infected individuals, levels of HTLV-1 virus in most infected individuals are often below the level of detection. The little data available give seemingly contradictory clues about the level of XMRV replication in infected individuals. The few sequences of XMRV genomes published to date display very little sequence variation, which implies limited cycles of replication in vivo. However, this notion seems to be contradicted by the observation of the reproducible isolation of infectious virus from the blood of CFS patients (4), along with reports of high levels of XMRV replication in cell culture (4, 10). Development of quantitative nucleic acid assays for XMRV, similar to those that directly quantify levels of plasma vire-mia in HIV-1 patients, will be required to characterize the level of viral replication of XMRV, and isolation of geographically separated isolates should help to determine the extent of genetic variation in this virus. If it is determined that XMRV has little genetic variation, but is significantly prevalent, it would indicate a lack of immune selective pressure on the virus. In addition, it would imply that humans had been exposed to an endogenous source of XMRV: if XMRV is present in the genome of an animal species, as X-MLV is in laboratory mice, the level of variation should be minimal.
Although XMRV has not yet been well characterized, much is known about the closely related X-MLVs and other members of their genus (gammaretroviruses). The life cycle and the associated diseases of these viruses provide important lessons for the study of XMRV. Although XMRV is the first human gammaretrovirus discovered, members of this genus are known to infect a variety of species including mice, cats, koalas, and non-human primates. Gammaretroviruses are primarily associated with leukemias and lymphomas but are also associated with other malignancies, immunodeficiencies, and neurological diseases.
Many cancers caused by gammaretroviruses are believed to involve events that occur when, during its replication, the virus inserts a copy of its genome into the cellular genome. If integration occurs near a proto-oncogene, transcription of that gene can be increased by the promoter and enhancer elements normally used by the virus to express its own proteins. Alternatively, viral insertions within a gene can disrupt expression, including tumor suppressor genes. Such alterations would confer a growth advantage on the cell, and could ultimately lead to cancer.
Although retroviral integration can occur throughout the genome, tumors induced by different retroviruses contain retro-viruses integrated into specific common sites in the chromosome, near proto-oncogenes or other cancer-related genes. For example, a percentage of malignancies caused by both MLV and another gammaretrovirus [feline leukemia virus (FeLV)] contain integrations near the proto-oncogene c-myc (11, 12). To date, XMRV integration sites have only been examined in a small number of prostate cancer tissue samples from XMRV-infected individuals. These samples did not show evidence of a common integration site near known oncogenes or tumor suppressor genes (13, 14). However, a number of the proviruses were integrated into transcriptionally active regions and several of the samples contained XMRV insertions near cancer-related genes and cancer breakpoints. Much more work will need to be done before it can be determined whether XMRV is a causative agent in associated prostate cancers and, if so, the mechanism through which it contributes to oncogenesis.
Retroviruses require specific receptors on a cell’s surface for viral entry. These receptors are different for different retroviruses. Early studies showed that the receptor for X-MLVs and the closely related polytropic MLVs (P-MLVs) is a transmembrane protein whose cellular function is unknown, termed xenotropic and poly-tropic retrovirus receptor 1 (Xpr1) (15–17). Recent studies indicate that XMRV also can use this molecule as a receptor: expression of Xpr1 in murine or hamster cell lines that cannot otherwise be infected by XMRV enabled the virus to infect and spread among the cells (6, 13). Previous work on MLVs may also provide insight into the regions of Xpr1 involved in interactions for XMRV: residues within two predicted extracellular loops of Xpr1 are required for cellular entry by both X-MLVs and P-MLVs (18, 19). Additional studies are necessary to determine whether, as is the case of HIV-1 and HTLV-1, the entry of XMRV into cells involves more than one molecule, whether the virus uses different types of receptors on different cell types, or both.
If studies of association with disease determine that XMRV is a public health concern, one important question will be whether antiretroviral therapy can impair XMRV replication in, or spread between, individuals. The answers to these questions will depend on the mechanism(s) of XMRV replication and the mode of virus transmission. At this time, the level of XMRV replication in infected individuals, the cell types infected, and the resultant pathogenesis of this infection are poorly understood. Clues to treatment may lie in the examination of other retroviral infections in humans and animals. HIV-1 infection results in immune dysfunction, owing to high degrees of virus replication and cytopathicity in the CD4+ T-cell compartment. Like HIV, MLVs can replicate to a high level in vivo, establishing high viral burdens, causing immune disorders through direct infection and proliferative disorders through site-selective DNA genome integration, dysregulating the expression of oncogenic gene products.
To date, antivirals directly targeting retroviruses have been primarily developed and tested against HIV-1, although AZT appears to have a beneficial effect in HTLV-1-infected individuals (20–22). Nucleoside reverse transcriptase inhibitors (NRTIs), which interfere with the ability of the virus to make a DNA copy of its RNA genome, would be expected to be most broadly reactive with XMRV. In addition, many of these drugs have already been tested against MLVs, which share a high degree of sequence identity with XMRV, particularly in the reverse transcriptase (RT) gene. Indeed, a recent study has demonstrated the effectiveness of one RT inhibitor, AZT, against XMRV replication in cell culture (23). However, the same study reported that another nine compounds licensed for treating HIV-1 did not inhibit XMRV replication. An obvious benefit of exploiting HIV-1 antivirals is that they are already tested and approved for clinical use (Table 1) [see (24)]. Clearly, the utility of such drugs will be more pronounced if robust replication of XMRV is intrinsic to its pathogenesis. If XMRV prevalence in humans is significant and it is found to be causally associated with disease, the extensive experience accrued by biomedical research community and pharmaceutical industry in HIV-1 antiviral drug development will be leveraged to develop specific inhibitors.
In Vitro Inhibitory Activities of FDA-Approved Antiretroviral Drugs Targeting HIV-1
Although antiviral medications have dramatically prolonged the lives of many individuals infected with HIV-1, attempts to develop an effective HIV-1 vaccine have proved difficult. One major difficulty has been the genetic diversity of HIV-1, including the extensive heterogeneity in the envelope gene (Env). This heterogeneity has made it difficult to generate envelope-specific antibodies that can neutralize different HIV-1 isolates. As mentioned above, initial data suggest that XMRV is less genetically diverse, which suggests that vaccine development will be easier than for HIV-1. In fact, effective vaccines have already been developed against the gammaretrovirus FeLV, which occurs naturally in domestic cats. Vaccines against FeLV have been commercially available for twenty years (25), and recent studies have shown that whole, inactivated virus vaccine is highly effective in preventing FeLV infection following challenge with infectious virus (26).
The association of XMRV with two widely disparate conditions, prostate cancer and CFS, raises the possibility that this virus may be widespread. Indeed, infectious virus has already been isolated from human blood (4). It is encouraging that genetic diversity among XMRV isolated to date is low, suggesting that development of a vaccine may be more feasible than it has been for HIV-1. Although the study of XMRV is in its infancy, knowledge of the life cycle, structure, and function of other retroviruses will likely be invaluable in the study of this virus. Indeed, by following the path well-worn by research on HIV and other mammalian retroviruses, research on XMRV might quickly provide inroads to abate its pathogenicity.
Acknowledgments
We thank Drs. Ferri Soheilian and Kunio Nagashima of the Advanced Technology Program, SAIC-Frederick, for the electron microscopy image of XMRV. We thank Drs. Maribeth Eiden, James Kaiser, Vineet KewalRamani, Christine Kozak, and Frank Maldarelli for input during preparation of the manuscript.
- Copyright © 2010
References

KyeongEun Lee, PhD, is a viral immunologist who works as a Research Fellow in Vineet KewalRamani’s group in the HIV Drug Resistance Program, National Cancer Institute–Frederick, in Maryland. She received her doctoral degree from the Department of Microbiology and Immunology, University of Texas Medical Branch at Galveston with Dr. Miles W. Cloyd studying HIV immunopathogenesis. She is currently interested in the mechanism of HIV nuclear entry, and has uncovered interactions between the virus and nuclear pore proteins. She is also pursuing studies examining xenotropic gammaretrovirus tropism and pathogenesis. E-mail leekye{at}mail.nih.gov.

Kathryn S. Jones, PhD, is a Principal Scientist with Science Applications International Corporation–Frederick, and works with Frank Ruscetti in the Laboratory of Experimental Immunology at the National Cancer Institute–Frederick, in Maryland. She received her doctoral degree from Albert Einstein College of Medicine in New York with Frank Lilly and did her post-doctoral fellowship with Anna Marie Skalka at Fox Chase Cancer Center in Philadelphia. The focus of her research is viral and cellular factors involved in transmission, persistence, and pathogenesis of HTLV-1. Recently, she has contributed to the discovery that dendritic cells participate in HTLV-1 transmission and to the characterization of the HTLV-1 receptor complex. E-mail joneska{at}mail.nih.gov; fax 301-846-7034.