Episodes in the Story of Physostigmine
“Within the infant rind of this small flower Poison hath residence, and medicine power”
— Romeo and Juliet, Act II, Sc. 3
Physostigmine (PS) is an alkaloid which first drew notice as an African ordeal poison. It later inspired studies by bold researchers who sometimes experimented on themselves, became a critical pharmacologic tool for the study of enzyme inhibition and neurotransmitters, found use as the first effective medicine for two serious diseases, and served as a model for development of new drug compounds. In addition, its synthesis was proclaimed a National Historic Chemical Monument in 1999. The highlights of PS’s history, reviewed below, are fascinating scientifically as well as in their human interest.
The Calabar Bean Metes Out Justice
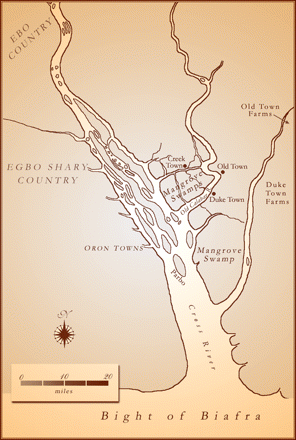
William Freeman Daniell, an English physician and botanist, came to Africa with an exploring expedition in 1845. His travels brought him to a region known as Old Calabar, at the delta of the Niger River, in what is now Nigeria. In 1846, Daniell delivered a paper, before the Ethnological Society, on the inhabitants of the region, describing their physiognomy, way of life, culture, and government. Justice, he reported, was administered by a court comprising the king and various chiefs. A person accused of a capital offense was brought before this court. If judged guilty, the suspect was forced to undergo an ordeal called “chopping nut.” The beans produced by an aquatic leguminous plant called esere were pounded in a mortar, then soaked in water until a milky white fluid resulted. The prisoner was made to drink this extract and was compelled to walk around until the poison took effect. Death was considered to be proof of guilt. If the prisoner happened to vomit before the poison began to work, he was adjudged innocent and set free (1, 2).
Could the Calabar bean really distinguish between the guilty and the innocent? It is amusing to read two widely different attempts at a rational explanation. Sneader (3), a historian of drug discovery, proposes an answer based on psychology. He suggests that a person confident of his innocence might have swallowed the potion rapidly, thus overwhelming his stomach and causing vomiting; whereas a guilty person, fearing to face the test, might have held the liquid in his mouth, facilitating buccal absorption of the alkaloid, or swallowed it slowly, thus avoiding emesis. A more cynical view is expressed in the 1926 edition of the United States Dispensatory (4). Because it was known that the beans, when fresh, have a more prominent emetic effect, the Dispensatory strongly hints that the priest or chieftain, who administered the ordeal, by judicious use of fresh or dried beans, could predetermine the outcome, thus shaping “justice” to his own ends.
Beans and Alkaloid: Early Studies
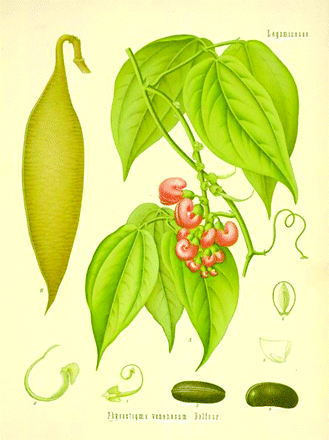
In the nineteenth century era of African exploration, Daniell’s report aroused great interest in his home country. Esere beans were sent back and planted in the Edinburgh Botanical Gardens, producing a striking-looking, climbing perennial with purple flowers and six-inch long seed pods. At that time, the Edinburgh Medical School was a major center of the study of pharmacology and toxicology (jointly known as materia medica). Robert Christison (1797–1882) became professor of materia medica in 1832, having previously gained a reputation in forensic medicine (5). As professor, he specialized in toxicological research. He administered various poisons to animals, observed their effects, and carried out post-mortem examinations of the organs. He also did not hesitate to experiment on himself.
Around mid-century, Christison turned his attention to the ordeal bean from Calabar, publishing his research in 1855. After first studying the effect on rabbits, he swallowed one quarter of a bean on an empty stomach. He experienced “a peculiar indescribable torpidity of the whole frame,” auricular fibrillation, and twitching of the voluntary muscles. Realizing that he had poisoned himself, he drank his soapy shaving water to make himself vomit, and had his son call in a professor of medicine to care for him. Christison recovered and lived to experiment on himself again. At age seventy-eight, he studied the effects of cocaine, reporting that it counteracted extreme fatigue and temporarily banished hunger and thirst (5). Thomas Fraser (1841–1920), Christison’s assistant and later his successor, conducted fuller studies on the Calabar bean, as described below.
Around 1861, Balfour published a comprehensive botanical description of the plant, naming it Physostigma venenosum. The stigma of the flower bears an unusual appendage which resembles a sac, or bladder, although it is actually solid. Balfour combined the Greek word for bladder, physo, with “stigma” for the Genus name (6). The species name, venenosum, derives from the Latin venenum, meaning poison (cf. venom).
Thomas Fraser was able to concentrate the active principle of physostigma beans, an alkaloid, into an amorphous powder which he called eserina. The alkaloid was first crystallized by Jobst and Hesse (Stuttgart, Germany) in 1864, and named PS (3). The name eserine has continued in use as well, as a synonym. Fraser described the action of both the concentrate and the pure alkaloid on the pupil, the central nervous system (CNS), heart, glands, voluntary muscles, and intestines. He discovered that they antagonized the action of atropine on the heart rate and its mydriatic effect on the pupil of the eye (7).
The mydriatic effect of atropine and related alkaloids had been discovered prior to 1820, but no means was available to counteract the dilation. Argyll Robinson, an Edinburgh ophthalmologist who was a friend of Fraser’s, learned of this from Fraser and successfully used PS in his practice (8). Ophthalmic application as a miotic thus became the first medical use of PS.
When applied to the eye, PS relaxes the ciliary muscles and improves outflow of aqueous humor, thus reducing elevated intra-ocular pressure. This makes it useful for controlling some types of glaucoma. Credit for establishing this therapeutic indication of PS is given to the German-Jewish ophthalmologist Ludwig Laqueur (1839–1909), who published in 1876. Laqueur, like Christison, experimented on himself, but in a more systematic way. Laqueur personally suffered from glaucoma and treated himself with PS, but his glaucoma eventually required surgery. He kept his condition secret because, as he later wrote, he wished to avoid people’s questions motivated by sympathy and curiosity, “repulsive to my modesty” (3, 9).
Pharmacology and Biochemistry
PS’s actions on the various organs and body systems were first discovered and reported by Fraser. PS increases the salivary, gastric, and bronchial secretions, increases intestinal tone and stimulates motility, slows the heart, dilates the peripheral blood vessels, and constricts the pupil of the eye (10). It counteracts the effects of atropine and the other Solanaceous alkaloids, as well as the paralytic effects of curare. These actions can be demonstrated in live animals or isolated tissues. The biochemical mechanism of these effects was elucidated by Otto Loewi (11). Loewi had earlier shown that the slowing of the heart produced by electrical stimulation of the vagus nerve did not arise from the electric current, but from a chemical substance secreted at the neuromuscular junction (Box 1). He named this material vagusstoff and was attempting to isolate it for further study. His efforts to obtain the substance from isolated organ preparations were stymied by its rapid disappearance, apparently owing to enzymatic action. The breakthrough came when Loewi discovered that this enzymatic inactivaton could be prevented by PS. He was then able, in 1926, to concentrate and isolate the vagusstoff, which proved to be acetylcholine. PS was thus the indispensable key to open the field of neurotransmitter research, one of the cornerstones of modern pharmacology and biochemistry (11).
Otto Loewi (1873–1961)
Otto Loewi was born in Frankfurt-am-Main, Germany. His early schooling emphasized Latin and Greek. His interests being in art history and music, he became an unwilling and dilatory medical student at Strassburg because of family pressure. This suddenly changed in the fall of 1894. Needing an attendance sign-off from a professor of internal medicine, Bernard Naunyn, he was forced to sit through a lecture by that professor. He was fascinated, and found an interest in experimental medicine. He did his thesis on inhibitors of the isolated frog heart, and received his MD in 1896.
Two years as a clinical physician left him frustrated with trying to treat patients dying of infections for which there was no effective treatment. He then decided to concentrate entirely on basic research. His studies on the effects of phlorizin, an inhibitor of glucose uptake, on nutrition in dogs with amino-acid mixtures replacing whole protein, and on glycogen mobilization upon injection of epinephrine, led to his appointment as professor of pharmacology in Graz, Austria.
Two crucial events in Loewi’s career occurred at 3:00 AM. In 1920, he was again studying the effects of drugs on the isolated frog heart and was stymied trying to prove that the action of nerves on their target organs was mediated by chemical substances. The wisdom of the time held that nerves act directly on the organs through the flow of electric current. One morning he jolted awake at 3:00 AM, having seen the answer to the problem in a dream. He rushed to the laboratory and dissected out two frog hearts, one with the vagus still attached, adding Ringer’s solution to keep the hearts beating. He applied electrical stimulation to the vagus, slowing the heartbeat till the heart stopped. He then transferred the Ringer’s solution from that heart to the second. Immediately the second heart slowed, showing that vagus stimulation must release a chemical substance. Loewi and his colleagues called the unknown substance vagusstoff, and later identified it as acetylcholine.
Loewi’s report met with skepticism, especially as the experiment was difficult to reproduce because of species differences among frogs. Finally, in 1926, at the International Congress of Physiology in Stockholm, Loewi conducted a demonstration in which the experiment worked eighteen consecutive times in the same pair of hearts. The concept of chemical transmission across synapses is now one of the cornerstones of neurobiology. In 1936, Loewi was awarded the Nobel Prize together with Sir Henry Dale, who had proved acetylcholine to be the transmitter between motor nerves and skeletal muscles.
Loewi was again awakened at 3:00 AM on May 11, 1938, the day after the anschluss of Austria by Hitler. Storm troopers broke in and carted him and his two younger sons (the only ones at home) off to a concentration camp, together with all the other male Jews of Graz. Eventually, he and the boys were allowed to leave the country, in return for turning over all his property, including the Nobel money, to the Reich. After short spells in Belgium and England, Loewi was offered a post as research professor of pharmacology at New York University School of Medicine. He came to the US in 1940, and became a citizen in 1946. He was thus able to continue his experimental work, and spent summers at the Woods Hole Marine Biological Station (23).
By its reversible inhibition of cholinesterase, PS intensifies all the effects of acetylcholine: its muscarinic effects on smooth muscle, glands, and the heart; nicotinic effects on skeletal muscle and the autonomic ganglia; and its CNS effects as well (10).
Current Medical Uses of Physostigmine (PS)
One application of PS is for atony of the smooth muscle of the intestinal tract and urinary bladder. Following abdominal surgery, the patient is often unable to have a bowel movement, to urinate, or both. The cholinergic action of PS stimulates the smooth muscle and counteracts this condition (12).
In glaucoma, the intraocular pressure is increased, and if persistent and sufficiently high, the optic disc, where the optic nerve joins the retina, may become damaged, resulting in permanent blindness. Of the three types of glaucoma (primary, secondary, and congenital), PS is helpful mainly in the primary type. Primary glaucoma is either narrow angle (acute congestive) or wide angle (chronic simple). In the former type, PS can control the acute attack, but surgery will be necessary. Wide-angle glaucoma is usually not improved by surgery, but can be controlled by continuous drug therapy (12). PS sulfate ophthalmic ointment and PS salicylate ophthalmic solution are available for use in glaucoma. However, PS has largely been replaced by another alkaloid, pilo-carpine, and by drugs acting through different mechanisms. Thus, beta-blockers (timolol), carbonic anhydrase inhibitors (dorzolamine), prostaglandins (bimatoprost), and adrenergic agonists (brimonidine) all reduce intra-ocular pressure and are options for the treatment of glaucoma.
Myasthenia gravis (MG) is a rare, devastating, exacerbating and remitting disease marked by weakness and rapid tiring of the skeletal muscles. A defect exists in synaptic transmission at the neuromuscular junction. It is now believed that MG is an autoimmune disorder in which the nicotinic acetylcholine receptors are attacked by the patient’s immune system, blocking access of acetylcholine to the voluntary muscles (12).
Although described and recognized as a clinical entity since 1877, MG lacked any effective treatment until Mary Walker made a discovery in 1934. Mary Walker was a Scottish-born single woman of a certain age, who had doggedly pursued her calling to become a physician, at a time when women were not welcomed in medicine. After serving with the Royal Army Medical Corps in Malta and Greece during World War I, she took a salaried position in the “Poor Law Service” at St. Alfege’s Hospital in an inner-city section of London. On June 2, 1934, in a letter to the Lancet, Mary Walker described Mrs. M., a 56-year old patient having an exacerbation of MG. First, Mrs. M. was unable to hold her shopping bag, and noticed her head falling forward. After a while she had trouble sitting up and had to remain in bed; first her jaw, then her left eyelid began to droop. Her speech became slurred, swallowing was difficult, and fluids were regurgitated through her nose. Could anything at all be done for this poor soul?
It struck Mary Walker that Mrs. M.’s symptoms were similar to the effects of curare. Because the known antidote for curare was PS, she gave Mrs. M. an injection of PS salicylate (1/60 grain, or about 1 mg). Within an hour, she saw improvement in the eyelid, the jaw, ability to swallow, and movements of the arm. With somewhat higher doses she attained greater improvement, and an effect lasting four to five hours. Walker found that oral administration of PS had almost no effect. She also, by way of controls, tried injections of water, strychnine, ephedrine, and acetylcholine with no effect. She proposed that the fatigability of MG was due to poisoning of the neuromuscular junctions (13–15).
Walker then collaborated with Roche, the Swiss manufacturer of PS, in searching for an orally active analog. By late 1934, she was able to treat Miss C., a 34-year old chambermaid, orally with neostigmine, a simple derivative of PS. Walker was elected to the Royal College of Physicians, and was later granted a medical degree from Edinburgh University, having submitted a thesis on her work on MG. She was offered prestigious posts at London teaching hospitals but, since these apparently required having an independent income, she had to keep practicing in salaried posts. In 2002, an annual Mary Walker Memorial Lecture was inaugurated at Queen Elizabeth Hospital, located at the site of the old St. Alfege’s (15).
There is another group of rare neurological conditions in which PS has proved somewhat helpful. These include Friedreich’s ataxia and other inherited ataxias. PS salicylate has been administered intravenously and as oral tablets. The improvement seen was not dramatic but enough to warrant further study with PS or other cholinergic agents (16). PS salicylate injection was granted official orphan drug status for treatment of Friedreich’s and other inherited ataxias in 1985, but research along these lines appears to have languished. In contrast, a condition which is unfortunately not rare is Alzheimer’s disease (AD). Some of the signs and symptoms of AD are attributable to a deficiency of cholinergic neurotrans-mission in the brain. As a potent cholinesterase inhibitor, PS has been tried for the treatment of AD (17). There was some effect, but its short duration of action was a problem. Conceivably, a long-acting dosage form could have been developed; instead, a patentable chemical derivative of PS—rivastigmine—was synthesized (see below).
Synthesis and Analogs of PS
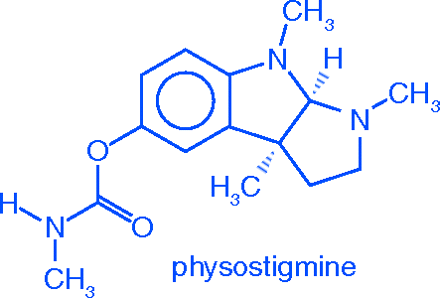
The structure of the PS molecule was not elucidated until 1925 (18). As alkaloids go, its structure is relatively simple—a 5-hydroxyindole with a pyrrolidine ring fused to it. However, before UV and IR spectrometers, mass spectrometers, and the other instrumentation routinely used today, alkaloid structures were determined by painstaking chemical degradation (“Abbau”) studies. In an interesting coincidence, the structure determination was carried out by Edgar Stedman and George Barger (18) at the University of Edinburgh, where the pioneering pharmacology studies had been done some seventy-five years earlier.
Efforts to synthesize PS were then initiated in two laboratories. One was led by Sir Robert Robinson (who became chemistry Nobelist in 1947) in England. The other was the laboratory of Percy Julian, an African-American chemist, who was a research fellow at DePauw University in Greencastle, Indiana. This seemingly uneven race was, surprisingly, won by the relatively obscure Julian. Robinson hit a snag at an intermediate step of the planned synthesis, a compound called eserethole. He simply could not convert this compound to the next intermediate. When Robinson published his results, Julian was surprised to find that the eserethole described by Robinson was completely different from the eserethole that he, Julian, had made. Despite Robinson’s stature, Julian published his results with confidence. Julian was readily able to convert his eserethole to the final intermediate, eseroline. Julian’s synthesis of PS could have been deemed complete at this point, since previous workers had demonstrated that carbamylation of eseroline yielded PS (19).
It happened, however, that Julian received a request from a pharmacologist for a sample of PS. To honor this request, Julian converted some of his eseroline into PS, and found it to be completely identical to the natural alkaloid. Thus, he was able justifiably to title his report “The Complete Synthesis of PS (Eserine)” (19). This small fact assumed greater significance some decades later, as follows.
Robert Burns (R.B.) Woodward (chemistry Nobelist 1965) gained great acclaim for the total synthesis of quinine, which had been originally reported by Woodward and Doering in 1944. However, in 2001, Gilbert Stork, at Columbia University, reporting on his stereoselective synthesis of quinine, pointed out that Woodward and Doering had not actually made quinine, but had accomplished only a “formal” synthesis of the alkaloid. They had gone as far as synthesizing the intermediate compound quinotoxin. Back in 1918, Rabe and Kindler, in Germany, had converted quinotoxin to quinine in three steps. Woodward and Doering relied on this work, without repeating it, in stating that they had done a complete synthesis. As Stork later put it “It’s a lawyer’s synthesis,” although with much justification because of the wartime situation (20, 21). It was this type of challenge and potential detraction that Julian avoided by going just the one extra step.
Percy L. Julian (1899–1975)
The son of a railway clerk and a teacher, and the grandson of slaves, Julian was born in Montgomery, Alabama. Although barred because of his race from his high school’s college preparatory program, he gained admittance to DePauw University in Indiana. He worked his way through college by digging ditches and waiting on tables at a frat house. He received a bachelor’s degree in 1920, making Phi Beta Kappa and class valedictorian. He earned his master’s in 1923 from Harvard, on a fellowship. After teaching for several years at black colleges, he received a Rockefeller Foundation grant in 1929, which enabled him to study at the University of Vienna, where he obtained the PhD in 1931.
In 1932, Julian returned to DePauw as a research fellow. Working with Josef Pikl, a colleague from Vienna, and several students, he produced, from 1932 to 1935, a number of high quality publications, culminating with the total synthesis of PS in 1935. He next addressed the extraction from soybean oil of stigmasterol, which is used in the production of sex hormones.
This author admits that, knowing nothing of DePauw, he at first considered it an unlikely place for organic synthesis of such high caliber. It turns out, however, that the teaching of chemistry at DePauw dates back to 1839.
Their chemistry department was established in 1881, and the chemistry major was first offered in 1896.
Although accepting African-Americans as students and giving Julian’s research group financial support, DePauw, as represented by its Board of Trustees, refused to give him a permanent faculty appointment. He therefore left in 1936, joining The Glidden Company in Chicago as director of research in the soya products division. There he developed soya and soya oil derivatives for use in textiles, paints, livestock and poultry feeds, candy, ink, cosmetics, and a foam used by the Navy in World War II to extinguish oil and gasoline fires. Using soya as a source for sterols, he also developed methods for making progesterone, testosterone and, in 1949, the then wonder-drug cortisone.
In 1954, Julian left Glidden to form Julian Laboratories, specializing in steroid research. He sold this business to Smith Kline & French in 1961, formed another independent research institute in 1964 and continued working in synthesis until his death. Julian had overcome the prejudices of his time to become a groundbreaking synthetic chemist, a successful research director, and a wealthy entrepreneur. He held ninety-four US patents and published more than fifty research articles.
Julian was very active in the civil rights movement during the 1950s and 1960s. In later years he received many honors, including appointment to the Board of Trustees at DePauw. In 1999, the American Chemical Society named the synthesis of PS at DePauw University a National Historic Chemical Landmark (24–26).
Even before the total synthesis of PS was accomplished, derivatives of the alkaloid were made in the hope of prolonging the duration of action and minimizing some of the undesirable cholinergic side-effects. Stedman and Barger prepared several simpler compounds, the most potent of which–miotine–was tested clinically, but proved not as good as PS. In 1931, the Roche firm, in Basle, introduced neostigmine. This compound, a dimethyl carbamate, is much more resistant to hydrolysis than PS, which has a monomethyl carbamate side-chain. As mentioned above, neostigmine was the drug used by Mary Walker to treat MG by oral administration. It is still in use for this disease, as well as for its stimulant action on the GI tract (11). Attempts to improve on neostigmine resulted, in the 1950s, in ambenonium, distigmine, and pyri-dostigmine. The last-named is still in use, and is recognized in the United States Pharmacopeia (USP) (11).
PS’s short span of action has rendered it useless for treating Alzheimer’s disease. A synthetic analog, rivastigmine, has been marketed, joining two other cholinesterase inhibitors, donezepil and galantamine. With an elimination half-life of 1.5 hours, rivastigmine also is not intrinsically long-acting. It is marketed in capsules and as an oral solution, both for twice-daily administration. More recently a rivastigmine trans-dermal patch became available for once-daily application to the patient’s back, upper arm, or chest (22).
Conclusions
The odyssey of PS, the chief alkaloid of the Calabar bean, has been traced from its starting point in the Niger delta, to Scotland, where it was first scientifically studied, to various lands in Continental Europe, back to Scotland for its structure determination, and finally across the Atlantic to the US for its total synthesis. Its history is associated with a number of adventurous medical scientists in the nineteenth century, and with three in the twentieth century who suffered discrimination because of their religion, gender, and race respectively. It became the first known treatment for two diseases which were previously untreatable and made possible discoveries which resulted in a Nobel Prize.
- Copyright © 2010
References

Stanley Scheindlin, DSc, holds a BS in pharmacy from Temple University and graduate degrees in pharmaceutical chemistry from Philadelphia College of Pharmacy and Science (now University of Sciences in Philadelphia). His academic research dealt with plant constituents and chemical interactions of vitamins. In his pharmaceutical industry career, he handled new drug formulation developments, and later regulatory affairs, presiding over the filing of about 100 generic new drug applications and two innovative drug applications. Now retired, his activities include volunteer work, consulting, and writing Reflections pieces for this journal. E-mail: stansch{at}verizon.net