Fractalkine/CX3CL1: A Potential New Target for Inflammatory Diseases
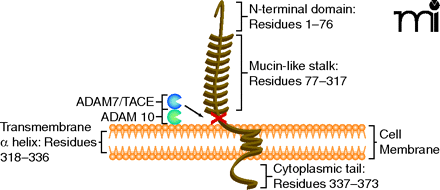
Figure 1
Membrane-bound fractalkine/CX3CL1. Schematic structure representation of fractalkine/CX3CL1, a membrane-bound chemokine consisting of a extracellular N-terminal domain (residues 1–76), a mucin-like stalk (residues 77–317), a transmembrane α helix (residues 318–336), and a short cytoplasmic tail (residues 337–373) (10, 57). Membrane-bound fractalkine/CX3CL1 is proteolytically cleaved by ADAM 10 and ADAM 17/TACE to generate soluble fractalkine/CX3CL1, which consists of the chemokine domain and the mucin-like stalk (11, 12, 58). The soluble form has chemoattractive activity for monocytes, natural killer cells, and T cells (9).



