Traveling Backward: De-differentiation to Stemness
For over thirty years, embryonic stem cells have monopolized stem cell research as the only totipotent regenerative cells capable of differentiation into all types of body cells. This view has changed over the last decade as ethical considerations have forced the pursuit of alternative regenerative cellular sources that possess molecular characteristics (“stemness”) similar to those of embryonic stem cells. In addition, regenerative medicine has focused on expanding the use of adult tissue–residing pluripotent mesenchymal stem cells including their harvest from bone marrow, adipose tissue, dental crest, and pancreatic islet cells (1–4). The differentiation of stem cells into mature body cells has been studied in detail; however, the reverse process of “de-differentiation” had been viewed as non-existent in “higher” vertebrates. One notable exception in vertebrates is the ability of some amphibians, especially urodeles, to produce progenitor cells via de-differentiation that can then contribute to the regeneration of organs or even body parts. In 2004, however, higher vertebrate myoblast cells were shown to de-differentiate in vitro into mono-nucleated cells with multipotent progenitor potential with the small molecule named “reversine” (5). Subsequently, in 2006, adult body cells of fibroblastic origin were shown to de-differentiate into induced pluripotent stem cells (iPS) through the retroviral transduction of four genes, Oct3/4, Klf4, Sox2, and c-myc (6).
There are, however, multiple ways to generate multipotent or pluripotent stem cells. For instance, one new way to generate mesenchymal stem cells from vascular endothelial cells through expression of a constitutively active mutant form of activin-like kinase 2 (ALK2) was recently presented by Medici et al. in association with fibrodysplasia ossificans progressiva (FOP)-dependent endothelial-mesenchymal transition (EndMT) (Box 1) (7). The identification of FOP-linked constitutively active ALK2 expression as the major regulator of vascular endothelial cell transition into multipotent mesenchymal fibroblast cells imposes similarities with the transgenic induction of induced pluripotent stem cells (IPs) generated from cells of fibroblastic origin (6, 7). Whereas the generation of iPS requires the four genes Oct3/4, Klf4, Sox2, and c-myc to de-differentiate cells completely into an embryonic phenotype, signaling activation of ALK2 by molecules of the bone morphogenic protein and activin receptor signaling family seems to be the missing link to de-differentiate cells from ectodermal-neuroectodermal origin into multipotent mesenchymal fibroblastic cells (6, 7).
EndMT and FOP Disease Pathology
Fibrodysplasia ossificans progressiva (FOP): An autosomal dominant disease that includes a mutation in the ALK2 gene (aminoacid exchange of arginine 206 into histidine; R206H) for expression of constitutively active form of the ALK2 receptor resulting in manifestation of heterotopic soft tissue ossification in response to acute inflammation.
EndMT: Transformation of endothelial cells from mesodermal origin (blood vessels) into mesenchymal cells (EndMT) can be found during endocardium development, artherosclerosis, cardiac and renal fibrosis. Includes inducible phosphorylation activation of the ALK receptor family (no known heterodimer formation of ALK2 and ALK5) by TGFbeta/BMP family type II receptors for Smad-pathway dependent activation of EndMT specific gene expression.
EndMT during FOP: Constitutively active (non-inducible) ALK2 receptor can interact with ALK5 and results in persisting phosphorylation activation of SMAD2 and SMAD5 for de-differentiation of vascular endothelial cells into pluripotent mesenchymal fibroblast cells that can then undergo chondrogenic-osteogenic differentiation during the progression of the FOP pathology.
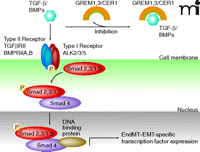
Transforming growth factor–beta (TGF-β) and BMP4 are the main regulators of normal (that is, not constitutively active) ALK-dependent EndMT induction (Figure 1) (7–10). In contrast to normally existing, inducible ALK-dependent downstream phosphorylation activation of Smad signaling molecules, EndMT in FOP is accompanied by persistent phosphorylation and activation of Smad2 and Smad5 through mutated, constitutively active ALK2. In detail, normal ALK2 was suggested to interact solely with and activate Smad5, thus, interaction of the ALK2 mutant with ALK5 was suggested to promote the additionally observed indirect phosphorylation activation of Smad2 in FOP associated EndMT (7). Correspondingly, persistent Smad2 and Smad5 signaling activation induces increased expression of transcription factors Snail, Slug, zinc finger E-box–binding homeobox-1 (ZEB-1), Smad-interacting protein-1 (SIP-1), and lymphoid enhancer–binding factor-1 (LEF-1) (7). Cells that undergo vascular endothelial cell trans-differentiation were also demonstrated to preserve the endothelial cell type markers TIE-2, VE-cadherin, CD31, and vWF; the mesenchymal markers FSP-1, alpha-smooth muscle actin, and N-cadherin; and the stem cell markers STRO-1, CD10, CD44, CD71, CD90, and CD117 (7). In addition, BMP7 and vascular endothelial growth factor (VEGF) were demonstrated to inhibit the FOP-dependent EndMT, suggesting that the disease pathology follows an endochondral ossification pathway that arises from the inhibitory action mediated by VEGF in EndMT (7).
The BMP/TGF-β signaling pathway and the Wnt/β-catenin signaling pathway can be inhibited by gremlins GREM1/3 or Cerberus 1(CER1) through heterodimer formation with ligands of the TGF-β/BMP family and prevention of TGF-β/BMP type II receptor/ligand interaction (25). Following TGF-β or BMP ligand binding and according phosphorylation activation of their corresponding type II signaling receptors, type I signaling receptors including receptors of the ALK family are phosphorylation activated and promote phosphorylation of Smad2,3 (TGF-β) or Smad1,5 (BMPs), respectively. Binding of phosphorylation-activated Smad2,3/Smad1,5 to Smad4 enables nuclear translocation and transcriptional activation of EndMT-associated transcription factors, via other DNA-binding co-factors. [Adapted with modifications from (26, 27)].
Similar to FOP-dependent EndMT, ALK-dependent signaling can be found in other EndMT and epithelial mesenchymal transition (EMT) associated pathologies such as scar formation and organ fibrosis of the skin, lung, heart, and other organs (11–14) (Box 2). ALK-dependent induction of EndMT has also been tightly linked to embryonic development, tissue regeneration, and tumor formation (15, 16). It is unknown whether other EndMT-associated pathologies include direct trans-differentiation of endothelial cells into a myofibroblastic cell type or undergo FOP-similar EndMT with ALK2-dependent de-differentiation into a mesenchymal precursor cell before differentiation into a myofibroblastic cell type.
EndMT versus EMT
Epithelial-to-mesenchymal transition (EMT) includes transformation of epithelial cells into mesenchymal cells and plays a role in gastrulation and embryonic development including neural crest and craniofacial development as well as wound healing, cataract, organ fibrosis and cancer development. Similar to EndMT, EMT uses the TGFbeta/BMP dependent signaling activation of the ALK/Smad-pathway.
Research on ALK2-mediated EndMT might provide the key for the development of medical therapies for a variety of EndMT or EMT-driven disease pathologies and to cancer (17). For instance, ALK2-targeted therapeutic intervention for EndMT or EMT pathologies might include the design of therapeutic miRNA, antibodies, or synthetic drugs that decrease ALK2-mediated signaling. In addition, therapies that induce constitutive ALK2 signaling activation might be beneficial for the treatment of other osteogenic diseases such as osteoporosis (18). Further, according to its role in osteogenic differentiation, the cell-based therapy with mesenchymal stem cells expressing constitutively active ALK2 in combination with novel bone matrices might improve bone-fracture healing and have an application during distraction osteogenesis [a procedure by which skeletal deformities, especially in long bones, are repaired by breaking the appropriate bone(s), gradually separating the fractured bone(s) (a process termed distraction), and permitting the formation of new bone in the gap] (19, 20).
In addition to delineating the molecular and cellular cause of FOP, this study (7) goes further to offer insights about dedifferentiation and the maintenance or induction of stemness. In the present case it is achieved with one gene and in the case of iPS with four genes, with Oct4 being virtually indispensable (6). This means that the path to stemness does not involve only one route, but possibly a networking of different pathways. In the newt, adult somatic cells can de-differentiate and create progenitor cells. This de-differentiation occurs in the case of limb regeneration (21), but in the case of lens regeneration there is also trans-differentiation of the pigmented epithelial cells to lens cells (22, 23). In both cases, Klf4, Sox2, and c-myc (three of the stemness factors) are expressed in the regeneration-contributing cells (24). It will be interesting to determine the role of Oct4 in these processes and to explore whether other signaling pathways participate (such as ALK2 signaling). Such comparative studies will eventually give insights about a stemness roadmap by pinpointing common mechanisms among different animals.
- Copyright © 2011
References
Andrea Hoffmann, PhD, is a Postdoctoral Fellow in the Tsonis Laboratory, Department of Biology, University of Dayton, Dayton, OH. She graduated with her master’s degree in biology from Universitaet des Saarlandes, Saarbruecken, Germany and received her PhD in biomedical sciences from Wright State University Dayton, OH. She has an extensive background in stem cell biology and regenerative medicine, and she is currently involved in studies related to cataract formation within the regenerating lens.

Panagiotis A. Tsonis, PhD, is a Professor of Molecular Biology and the Director of the Center for Tissue Regeneration and Engineering at the University of Dayton, Ohio. He is very interested in many issues of tissue regeneration, the mechanisms of transdifferentiation, and induction of regeneration in mammals. E-mail panagiotis.tsonis{at}notes.udayton.edu; fax 937-229-2021.