
- Institution: Stanford Univ Med Ctr Lane Med Lib/Periodical Dept/Rm L109
- Sign In as Member / Individual
Emerging Roles of TACE as a Key Protease in ErbB Ligand Shedding
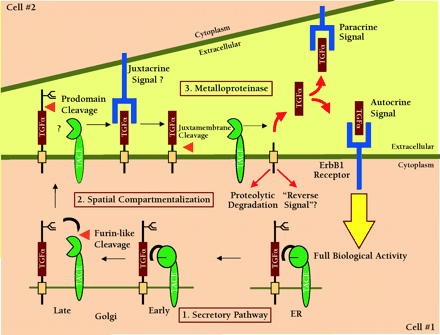
TACE-dependent proteolysis of the proTGFα extracellular domain as a regulatory event in ErbB1 receptor signaling. Genetic and biochemical data indicate that the release of soluble TGFα acting either as an autocrine or a paracrine signal is essential to achieve full biological activity in vivo. Data from Sunnarborg et al. suggest that TACE may also be involved in the N-terminal prodomain processing of proTGFα (16). Many questions still remain about the specificity and regulation of TACE as general ErbB ligand “sheddase.” Some of these questions can be divided into three general areas. 1. Secretory Pathway. How is the biosynthesis and activation of TACE regulated and can active TACE cleave substrate during transit through the secretory pathway? 2. Spatial Compartmentalization. What is the trafficking of TACE, what membrane compartments is it localized to and how is access of TACE to substrate regulated? 3. Metalloproteinases. What is the specificity of TACE for other ErbB ligands? Is the catalytic activity of TACE regulated? What is the role of other metalloproteinase(s) in ErbB ligand shedding?


