What IS training in the Pharmacological Sciences?
- Peter C. Preusch, PhD, Program Director

NIGMS Pharmacological Sciences Training Grant Meeting
On August 8–9, over 150 scientists met in Bethesda, MD, under the auspices of the National Institutes of Health, to address
concerns regarding training programs in the pharmacological sciences. Participants included most of the currently funded training
grant directors in the pharmacological sciences; members of the Biomedical Research Training Initial Review Group of the National
Institute of General Medical Sciences (NIGMS); and representatives from relevant scientific societies, industry, government,
and over seventy-five research institutions. Speakers included: Gordon Amidon, Kim Brouwer, Alan Buckpitt, Bryan Cox, David
Eaton, Robert Gould, James Hogle, Cynthia Kuhn, Lee Limbird, David Mangelsdorf, Richard Weinshilboum, and Thomas Westfall.
(See http://www.nigms.nih.gov/news/meetings/pharmscitraining.)
Following passage of the National Research Service Act (NRSA) in 1974, NIGMS established interdisciplinary training programs in four areas: Cell and Molecular Biology, Genetics, Systems and Integrative Biology, and Pharmacological Sciences (PS). The PS program supported interdisciplinary training in pharmacology, toxicology, medicinal chemistry, pharmaceutical chemistry, and related disciplines. Programs in Molecular Biophysics, Biotechnology, Chemistry/Biology Interface, and Bioinformatics training were begun in 1988, 1990, 1994, and 2001. The number of PS trainees grew to a peak of about 290 students in 1992, but has since declined to 204 in FY2002. The number of funded PS programs has also decreased. This decline plus a number of other concerns motivated NIGMS to organize the meeting.
The objectives of the meeting were to assure scientific diversity in the research training supported nationwide through the NIGMS portfolio of PS training grant awards and to define the core knowledge that distinguishes PS training programs from other programs supported by NIGMS. In addition, the meeting provided a venue for current trainers, applicants, potential applicants, reviewers, and NIH staff to exchange general ideas outside of the confines of the normal application and peer review process. The meeting was designed to raise ideas for discussion and generate suggestions that might be considered by individual programs. The following topic sections blend comments of the speakers, results from breakout sessions, and comments by NIGMS staff. There is little actual written policy concerning most of these issues. Rather, practices have arisen through dialog between applicants, reviewers, and NIH staff, and it is these practices the meeting sought to examine.
What is core training in the pharmacological sciences?
There was consensus among the meeting participants that pharmacology is the core discipline in which all PS trainees should receive instruction. There was remarkable consensus that the core subject matter of pharmacology remains the principles of pharmacokinetics and pharmacodynamics. Pharmacokinetics was defined as including not only the collection and modeling of concentration-versus-time data, but also an understanding of the mechanisms underlying the absorption, distribution, metabolism, and elimination of drugs. Pharmacodynamics was defined as including not only dose-response data, but also the mechanisms of drug action, receptor–ligand binding, signal transduction, and enzyme inhibition. Most programs also provide some training in therapeutic categories, although some do so as part of advanced courses or electives, rather than as part of the basic pharmacology curriculum taken by all students.
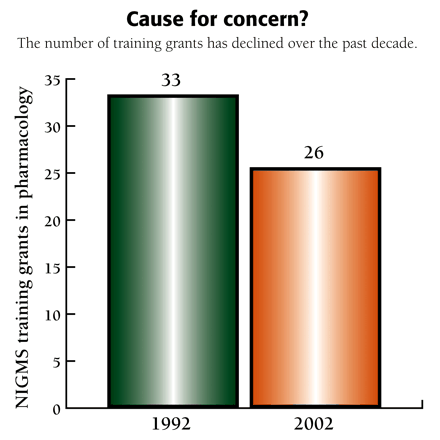
Cause for concern?
The number of training grants has declined over the past decade.
What is state-of-the-art training and how can it be accomplished?
There was consensus that cutting-edge research training is dependent on the research activity of the faculty members at any given institution. Team teaching by large numbers of research-active faculty in their areas of expertise was suggested as a mode of instruction that assures inclusion of state-of-the-art technology and information. However, a weakness of team teaching may arise from a reluctance of faculty members to cover important material that is not within their areas of expertise. The administrative setting for instruction (e.g., voluntary teaching in an interdepartmental program versus departmental teaching obligations) can influence what material is taught. Core research facilities also can provide training in new technology, provided that students are given the chance to use the facilities in their research, preferably with hands-on experience. Journal clubs and seminars emphasizing the primary literature, rather than lectures alone, may also be an effective way to introduce new techniques. Clearly, the incorporation of genomics, proteomics, and bioinformatics into biomedicine has become essential and should be experienced by students in PS programs.
What is the proper balance between molecular/cellular and systems/integrative pharmacology?
The need for systems and integrative training in pharmacology was repeatedly mentioned by speakers from academia, industry, and government (i.e., the FDA). From the industry perspective, systems and integrative pharmacology training means experience in the use of in vivo animal models. In particular, the ability to think critically about the relevance of animal models to the human clinical situation, rather than mere technical skills in a particular experimental model, is viewed as essential to training. This ability is important in all stages of drug discovery and development, from target selection through safety evaluation. From the academic perspective, whereas systems and integrative pharmacology may include whole animals, isolated organs, or tissues, pharmacologists should be trained to integrate information at all levels, from the molecular to the whole organism, in order to understand system responses in disease and therapy.
Generally, there was agreement that both molecular/cellular and systems/integrative approaches are needed. Several examples were presented from drug discovery programs. In academic research, the integration of new molecular/cellular technologies with human clinical observation was viewed as a growing need in order to fully exploit the new pharmacogenetics and pharmacogenomics data; both systems and reductionist approaches are needed. The current research environment was described as undergoing a paradigm shift, where systematic data-gathering supplements—or even anticipates—traditional hypothesis testing. However, the importance of connecting novel science to its fundamental roots was also emphasized.
What are some possible remedies to the decline of in vivo pharmacology?
The decline of in vivo animal pharmacology in academia, and concerns about the consequences of this decline, have been raised previously and were reviewed at the meeting (1, 2). Efforts to reverse this decline include the Integrative Medical Sciences Initiative, activities of the ASPET Government and Public Affairs Office and other professional societies (3, 4), formation of ASPET’s new Division of Systems and Integrative Pharmacology (5), and meetings including NIH staff (6). Inclusion of the words “systems and integrative” within both appropriations language (7) and comments by the new NIH director may be taken as an encouraging sign (8).
Addressing the lack of in vivo training will depend primarily upon the faculty hired by universities over the next few years. It will also depend on the ability of faculty to obtain funding for in vivo studies. In this context, the current reorganization of the NIH Centers for Scientific Review is seen as both an opportunity and a possible cause for concern: Appropriate study sections must be formed so that their members can effectively review in vivo research. More than ever before, grant applicants must be able to compellingly argue that in vivo efforts will likely yield good and new scientific discoveries. Animal rights activism has clearly had an impact on in vivo research. It is important that both medical and graduate education provide ethical training in the appropriate use of animals in research, and that researchers develop advocates able to articulate the importance of animal work to human health.
It is the rare individual who will be able to consider the breadth of biomedical science in depth. For this reason, much work in academia—and almost all work in industry—makes use of teams. There needs to be a cadre of trainees that represent expertise throughout the spectrum of pharmacology, from molecular to whole organism studies. In addition, it would benefit all trainees to learn the language(s) needed to communicate with other team members, including the language of the in vivo experimentalist. NIGMS staff noted that a small increase in the number of students receiving in vivo training at a few of the currently funded PS programs, or the successful (re-)establishment of a few programs focused on systems and integrative training, would immediately and substantially address the present problem. Greater advantage could also be taken of other NIGMS predoctoral training programs to support in vivo training, particularly the Systems and Integrative Biology and Medical Scientist Training Grant programs. Other suggestions included utilization of industrial experiences, greater use of the NRSA F32 postdoctoral fellowship program, encouraging faculty members to teach outside their areas of expertise, and reaching out around the campus to other pockets where systems and integrative science may be taking place.
“Is that real pharmacology?”
—Adherence to traditional pharmacological definitions carries the risk of suffocating the discipline, according to Vanderbilt’s Lee Limbird.
What can be done to increase the scientific diversity of PS training opportunities?
Multidisciplinary approaches to pharmacology may on occasion elicit the question from purist practitioners, “Is that real pharmacology?” Purist appraisals, however, were viewed as counterproductive, equivalent to putting pharmacology under a bell jar: safe, but suffocating. As the NIGMS program description points out, the PS training program does not exist to train pharmacologists per se, but rather has historically included a broad range of disciplines with homes in schools of pharmacy, arts and sciences, agriculture, and medicine. Diversity within the overall portfolio of PS training grants is desirable and should arise naturally from the variety of strengths at various institutions.
Schools of pharmacy have been important in bringing medicinal chemistry and pharmaceutical chemistry into training programs. Schools of pharmacy may also provide access to clinical research through the training of PharmD students (9). Depending on the institution, toxicology may be offered in the school of medicine, school of pharmacy, or school of public health. Although the fields of pharmacology and toxicology have diverged considerably over the past several decades, they share a common core of concepts, and historically both have been supported by NIGMS training grants. Issues concerning the balance between molecular/cellular and systems/integrative studies are very similar in toxicology. Although NIEHS now supports much of the training in toxicology, drug-relevant toxicology could be appropriately included in PS training programs.
The question of scientific diversity encompasses the questions regarding the importance of systems and integrative studies. In vivo research training, particularly in the area of pharmacokinetics, is currently stronger within schools of pharmacy than in schools of medicine. Schools of veterinary medicine and departments of animal science would seem reasonable places to find additional in vivo animal research (10, 11). Pharmacology and toxicology training within veterinary schools are distinct because of the emphasis on comparative and integrative approaches. Human subjects may represent the ideal integrative system and the inclusion of clinical research within the PS training program by engaging both MD and PharmD students should be explored (12). Bridge building between the NIGMS predoctoral PS program and the postdoctoral training programs in clinical pharmacology might also be encouraged. At relatively few institutions across the country is it possible to conduct pharmacological research from cells to mice to pigs to man. Those few institutions that maintain such strengths are encouraged by NIGMS staff to include them in their PS training programs.
PS programs at institutions that have good relationships across school boundaries have benefited by being able to recruit larger and more diverse faculty and student pools, and hence, more stipend support from NIGMS. Traditional student pools for pharmacology programs do not appear to be growing, and PS programs must compete with many other NIGMS training programs for students with molecular/cellular interests. PharmD and DVM students, as well as PhD students, within schools of pharmacy and veterinary medicine may represent an underutilized pool of potential students for PS programs. Obstacles to recruiting such students are similar to those faced in recruiting MD students: long training times, debt burdens, and competition with clinical practice. Debt relief programs, dual degree training programs, and early research experiences could thus be useful in recruiting from these pools.
At the other end of the training pipeline, the lack of pharmacology undergraduate programs has been often raised as a reason for the difficulty of recruiting graduate students into pharmacology. There are a few such undergraduate programs (13). Perhaps a few additional programs could be started. Certainly, pharmacology courses at the undergraduate level could be offered on nearly every undergraduate campus.
Would incorporation of a clinical experience within the PS graduate program be helpful?
It was agreed that providing a clinical orientation to PS graduate students via a course (14–16) or rotation would produce PhD graduates with a greater appreciation for the conduct of clinical research and a better understanding of the interplay between laboratory research and clinical research. In addition, more extensive training for biomedical professionals (e.g., MD, PharmD, DVM graduates) was also felt necessary.
The resources (e.g., faculty, financial incentives, and infrastructure) necessary to provide PS trainees with clinical experience need to be considered. NIGMS policy permits the appointment to PS training grants of dual degree candidates pursuing PhD-credited activities and also professional postgraduates who choose to pursue a PhD program. However, institutions may need to find supplemental sources of non-federal funds to make stipend levels competitive with other career options.
Would incorporation of an industrial experience within the PS training program be helpful?
There was agreement that industry experiences, particularly as a substitute for one academic rotation, would be a useful option for students. Industrial rotations should be encouraged, but should not be mandated. The arrangements need to be approached carefully on a case-by-case basis to match student and industry interests. Completion of a large part of the thesis in an industrial laboratory may be possible, but presents some challenges. For example, expectations regarding publishability of results need to be well-defined in advance. NIGMS policy permits students pursuing research off-site to be appointed to a training grant provided that they are still able to participate in those activities of the training program (e.g., seminars, journal clubs) pursued by students working at the grantee institution. Adjunct faculty in industry may be listed as potential mentors in the training grant application.
How have changes in medical education impacted graduate education and vice versa?
The majority of pharmacology programs no longer require—although many of them still allow—their graduate students to take medical pharmacology along with medical students. In many schools, medical education has shifted from a discipline-based approach to an organ systems–based approach, and often there is no longer a single coherent medical course given in pharmacology. Solutions to the loss of pharmacology courses have included: 1) having students attend portions of the integrated medical curriculum dealing with pharmacology; 2) separate problem sets for graduate students and medical students that test different types of pharmacology knowledge; 3) taking advantage of pharmacology taught for allied health students; and most commonly, 4) creation of a separate graduate course in the principles of pharmacology. Advanced courses, electives, and journal clubs have further been used to cover selected therapeutic topics and clinical problems. Physiology courses have been similarly affected. In some programs, an integrated course of physiology and pharmacology has been developed. Elimination of medical pharmacology has also limited the teaching opportunities for students. Extra outreach efforts are therefore needed to place students into teaching positions. Although there has been some concern that if PS trainees are not exposed to medical pharmacology they will not be prepared to teach pharmacology to medical students later (17), this concern was not endorsed by program directors at the meeting. It is noteworthy that many of the speakers, luminaries in pharmacology, introduced themselves by saying that they did not get their PhD degree in pharmacology.
What are common elements of PS training programs?
During the meeting, details of ten programs were presented as examples. Although each program was different, most shared common elements. Common training program activities include: recruitment of students; appointment of students to the training grant; mentoring and monitoring student progress; rotations prior to selection of thesis laboratory; and didactic components such as core courses and electives, cumulative and/or entrance to candidacy exams, thesis proposal and/or NIH-style practice proposal, journal clubs, seminars, social activities, retreats, student seminars, poster sessions, and opportunities to attend scientific meetings.
Common faults of inadequate proposals include: poorly defined administrative structures; lack of process and/or criteria in faculty selection; faculty turnover; failure to address mechanisms of student monitoring; weaknesses in proposed curriculum (e.g., inadequate preparation in physiology; limited scope of coursework; inappropriate number of course requirements, and failure to anticipate time required for completion of program); lack of multidisciplinarity; lack of flexibility; inadequate program definition; and insufficient student pool.
The pivotal factor in many cases will be the student pool and the anticipated outcome of the training experience. How strong is the student pool and how “deep” is it? What is the record of past trainees and how are the current students benefiting from the training they receive? University-wide umbrella recruiting and first-year core programs can be advantageous, but may require a period of adjustment. In many cases, the university covers all first-year stipends, and students can be appointed to the training grant in the second year, when their commitment to the program is better assured. First-year coursework should prepare trainees for instruction in pharmacology by the second year. However, care must be taken to be sure the incoming class contains students who will eventually choose the PS training program. The student pool may be drawn from across the entire institution so as to ensure program interdisciplinarity and a dependable pool of trainees. Thus, realistic estimates of the number of interested, eligible students and careful plans for how the students will be selected, mentored, and monitored must be presented. A very experienced reviewer made the following comments: Preparation of a good proposal is vital. A poor proposal may fail to document what is actually a strong training program. In preparing a proposal, carefully read the program announcement and related information available on the NIGMS Web site regarding review. Reread the summary statement from any previous reviews. Use of the recommended table formats will make the reviewers’ job easier.
What are the outcomes of PS training? What training is needed in academia and in industry? What is important and what is not?
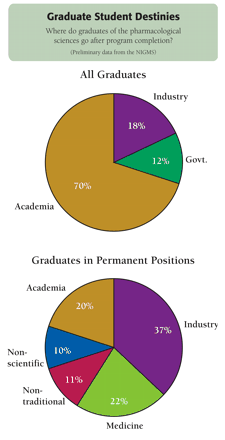
Based on responses from PS training program directors, most graduates have gone on to postdoctoral positions in academia (70%), industry (18%), or government (12%). Of the graduates assumed to be in permanent positions, 37% were in industry, 20% in academia, 22% in medicine, and 11% in nontraditional careers; only 10% left science altogether. Interestingly, academic and industrial speakers are alike in the skills and talents that they wish to develop in trainees: a wide range of research experience; the ability to work collaboratively; problem-solving and life-learning skills; and the ability to respond productively to constructive criticism. Technical skills and specific subject area knowledge are viewed as secondary goals. Evidence of accomplishment as documented by peer-reviewed publications is important. Although participants from industry emphasize the need for more in vivo training, people able to integrate in vitro and in vivo research are highly valued above all else. Also of value to industry are an appreciation for the meaning of the term “safe and efficacious,” and a passion for the goals of drug development.
Among a panel of recent PS graduates, training in basic physiology, pharmacokinetics, and pharmacodynamics was regarded as fundamental to their current activities. Other valued experiences from graduate training included opportunities to give presentations, attend scientific meetings, solve problems independently, and develop general professional skills. Courses based on research papers and journal clubs, and examination of classic papers as well as cutting-edge research literature were preferred. Many of the panel members had taken regular medical pharmacology and found it to be useful, but not essential. No one mentioned having learned about any specific drug class as important to their current employment. Some felt that teaching experiences were important. Generally, teaching opportunities were made available to those wanting them. Industrial internships as a rotation option would have been welcomed by some.
Closing Comments by NIGMS Staff: Past and Future PS Training Programs
Since 1974, a total of forty-one different PS training programs have been funded by NIGMS. Almost half of the currently funded training programs were initiated in the initial three-year ramp-up of programs following passage of the NRSA. An almost equal number of programs initiated in the period 1974–1977 have now ended. The recent decline in the number of funded programs suggests a need for reexamination and rejuvenation of the portfolio of NIGMS-supported programs.
A total of seventy-nine institutions are currently supported by one or more of the ten types of NIGMS predoctoral (PhD or MD/PhD) training grants. Eighty percent are located on campuses with a medical school, a school of pharmacy, or a school of veterinary medicine (i.e., academic units that could logically provide the base for development of a PS training program). Of those schools, only about forty percent received PS training grant awards in FY2001, suggesting a substantial untapped pool of research-intensive institutions from which new PS programs might arise. Furthermore, there are another eighty-eight institutions nationwide that currently receive no NIGMS training grants, despite the presence of a school of medicine (fifty-six institutions), school of pharmacy (forty-nine institutions), and/or school of veterinary medicine (fourteen institutions). Some of these eighty-eight institutions might prove to be competitive for a PS training grant award. Potential applicants are urged to contact NIGMS staff to discuss their ideas.
Acknowledgments
NIGMS wishes to thank the members of the meeting organizing committee for many helpful suggestions as well as their presentations and participation in the meeting. Our appreciation goes out to: Gordon Amidon, Bryan Cox, Robert Gould, James Hogle, Cynthia Kuhn, Lee Limbird, David Mangelsdorf, Richard Weinshilboum, and Thomas Westfall.
- © American Society for Pharmacology and Experimental Theraputics 2002

Peter C. Preusch



