
- Institution: Stanford Univ Med Ctr Lane Med Lib/Periodical Dept/Rm L109
- Sign In as Member / Individual
Localization of Receptor-Mediated Signal Transduction Pathways: The Inside Story
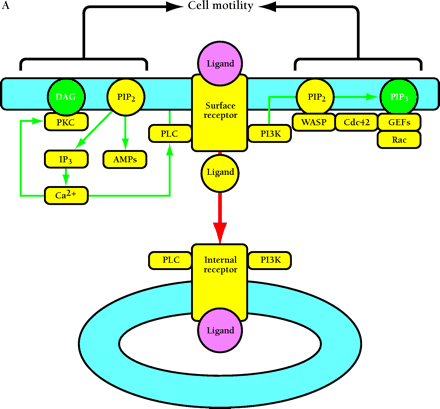
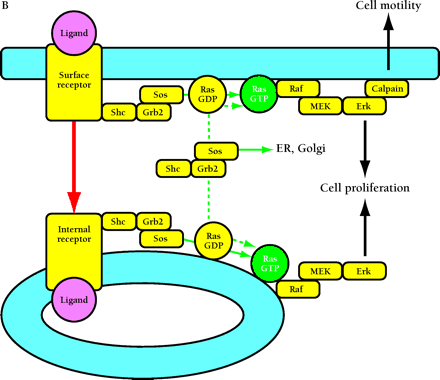
Compartmentalized activation of signaling pathways. A. Restricted localization of PI(4,5)P2. Surface and internalized receptors engage and phosphorylate PLC and PI3K, but the lipid substrate PI(4,5)P2 (PIP2) is not accessible to internalized receptors. Thus, only surface receptors mediate PIP2 hydrolysis by PLC, forming 1,2-diacylglycerol (DAG) and inositol (1,4,5)-trisphosphate (IP3) and releasing actin-modifying proteins (AMPs). IP3 releases intracellular calcium, which cooperates with DAG to activate protein kinase C (PKC). PI3K phosphorylation of PIP2 is also restricted to the cell surface. The product PI(3,4,5)P3 (PIP3) recruits many signaling proteins, including guanine nucleotide exchange factors (GEFs) that act upon the Rho family GTPases Rac and Cdc42. Rac•GTP mediates membrane ruffling, while Cdc42•GTP and PIP2 cooperatively activate Wiskott-Aldrich syndrome protein (WASP), which regulates actin polymerization. All of these signals play important roles in localized cell motility processes. PIP2 is also required for multiple stages of clathrin-coated pit endocytosis. B. (See next page.) Global activation of Ras and Erk. Ras•GTP loading is stimulated by receptor recruitment of Grb2–Sos complexes, mediated in many cell types by tyrosine-phosphorylated Shc. Both surface and internalized receptors bind to and phosphorylate Shc, recruit Grb2–Sos, and participate in Ras•GTP loading. It was recently confirmed that endosomes contain Ras, and Raf, MEK, and Erk are activated at each location. Activated Erk is released into the cytosol, and phosphorylation of cytosolic and nuclear Erk substrates is required for cell cycle progression. Erk activated at the plasma membrane is specific for activation of calpain, a calcium-dependent protease important for modulating cell adhesion and motility. Other recent evidence indicates that Ras can be activated in compartments that lack activated receptors, such as the ER and Golgi, presumably by Shc–Grb2–Sos complexes recruited from the cytosol.


