What is the Value of Measuring MTA-1s in Breast Cancer?
Described in the recent paper by Kumar et al. is an elegant study of metastatic tumor antigen-1 (MTA-1) and its splice variant (MTA-1s) in adenocarcinoma of the breast (1) . Perhaps the study itself was not intended to answer questions of clinical value, but instead to investigate possible mechanisms of cytosolic retention of ERα in breast cancer cells. Nevertheless, the background information cited in the paper, namely, that nuclear ERα is a good prognostic marker for breast cancer, and that high expression of MTA1 is associated with invasiveness, does set the tone and raises expectations that issues related to breast cancer will be addressed. The conclusion of the study is that MTA-1s is expressed in the cytoplasm of ERα -negative breast cancers—that is, cancers that do not express any nuclear-localized ERα —and that MTA-1s sequesters ERα in the cytoplasmic compartment thus preventing its transcriptional activity.
In a previous study, Kumar et al. showed that MTA-1 is a potent corepressor of estrogen receptor element- (ERE)-dependent gene transcription, because it blocks the ability of estradiol to stimulate ER-mediated transcription. MTA-1 also directly interacts with histone deacetylase-1 and -2 and with the activation domain of ERα (2) . Furthermore, overexpression of MTA1 in breast cancer cells results in increased invasiveness and anchorage-independent growth. In other words, ER-mediated transcription is blocked by both MTA1 and the MTA splice variant, MTA-1s.
How does this information help us to understand breast cancer and its treatment?
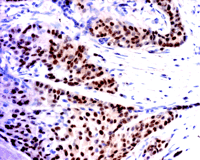
It is known that the presence of nuclear ERα in breast cancer (Figure 1⇓) is a good prognostic indicator of whether a patient will respond to antiestrogen therapy. (A good response being that, upon tamoxifen treatment, the patient will be in remission for five years or more.) Tamoxifen is an antiestrogen used clinically to block the actions of estrogen. It binds to the ligand-binding site in ERs and prevents the binding of estradiol. When tamoxifen is bound to ER, the receptor assumes a conformation that does not permit its interaction with coactivator proteins, and the receptor is transcriptionally inactive. Clinicians are also very clear on one point: if a particular breast cancer has high ERα expression, the prognosis is much better than if the cancer is ERα negative, whether or not the patient is treated with tamoxifen. It appears that an ERα -expressing cancer is in a better-differentiated, less malignant state and that estrogen is still an important growth factor, as it is in normal breast tissue. Antiestrogens can be used successfully to inhibit growth of such tumors. In an ERα -negative cancer, growth is regulated by factors other than estrogen.
If a splice variant of MTA1 is expressed in the cytosol and if it sequesters ERα into this compartment, ERα would be transcriptionally inactive. Would this not be similar to blocking ERα action with an antiestrogen? If the cancer cells have ERα in their cytoplasm but not in their nuclei, the presence of MTA-1s might account for the sequestration of ERα in the cytoplasm. If MTA-1s is expressed in cancers that do not express ERα in the nucleus, does this mean that it is a marker for less well-differentiated cancer? Or does it mean that a well-differentiated cancer can appear to be ERα -negative because its ERα is sequestered in the cytoplasm? Alternatively, does it mean that this cancer has already escaped growth regulation by estrogen?
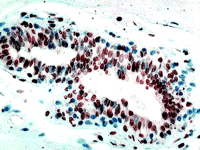
Two factors have to be considered before we focus too much attention on the clinical usefulness of MTA-1s. The first is that all proteins with LXXLL motifs—and there are hundreds of these—will compete with ERα for binding to MTA-1s. ERα would have to be sequestered in the cytoplasm by MTA-1s, in order for MTA-1s to exert a physiologically important influence on ERα function in breast cancer cells. The conundrum is that the expression level of ERα is very low in cells, much lower than those of the glucocorticoid receptor (GR), ERα , or progesterone receptor (PR). Thus, we might expect many other LXXLL-containing proteins to successfully compete with ER for binding to MTA-1s in the cytoplasm. The second factor, which must be included in the overall equation, is the expression of ERβ (Figure 2⇓), which was mentioned in the Kumar study. In many breast cancers, the amounts of expressed ERβ are higher than that of ERα and would compete with ERα for available cytosolic MTA-1s. Yet, it is clear that responsiveness of breast cancer to tamoxifen is related to expression of ERα and not ERβ . Does this mean that, in MTA-1s-expressing cancers, expression of ERβ might allow more ERα to remain in the nucleus and thus restore responsiveness to tamoxifen?
In order to put all of these questions into perspective, we need to know whether accumulation of MTA-1s in cytosol is a primary event in the development of invasiveness and resistance to tamoxifen, or whether it is one of several changes that occurs as cells continue to replicate and become more malignant. A large number of metastasis-associated genes have been identified (3) . Toh et al. originally found that the MTA gene in this study was more highly expressed in metastatic than in nonmetastatic cell lines (4) . This gene is expressed in all normal tissue and is thought to be involved in cell proliferation. It is also expressed in gastrointestinal and esophageal cancers (5, 6) . If expression of MTA-1s is the key factor in sequestration of ERα in cytosol, and this process switches on a more invasive phenotype, then the clinical value of the Kumar et al. (1) study becomes clear. It might be possible that by blocking the activity of MTA-1s in breast cancer cells, we could prevent the sequestration of ERα in the cytoplasm and halt the progress of the cancer phenotype toward more a malignant form. In this case, clinicians would have a new contribution to the arsenal of adjuvant treatment of breast cancer.
It might be too early to judge the clinical significance of MTA -1s expression. In the paper by Kumar et al. (1) , there are thirty-one breast cancer samples. Although most of the ERα -negative cancer samples have higher levels of MTA-1s as compared to ERα -positive samples, there are three ERα -negative samples with lower amounts of MTA-1s, and the MTA-1s levels in ERα -positive samples vary widely. No correlations were made between ERα expression and tumor differentiation or menopausal status of patients, both of which do influence ERα status of tumors. Perhaps, in the future, we can look forward to a random, blinded study where the expression of MTA-1s in human cancer samples is compared with invasiveness and state of differentiation of each tumor. In addition, in order to illustrate that it is the ERα -negative cells that express MTAs, colocalization of MTAs and ERα in cancers is necessary, because in ERα -positive samples, there are many cells that do not express ERα .
At present, there is no evidence that the measurement of MTA-1s will act as a prognostic marker or as an index for antiestrogen treatment. But it is reassuring to know that these MTA-1s positive patients, who would not normally be treated with tamoxifen, should not be treated with tamoxifen.
ERα expression. ERα is expressed in about 70% of breast cancer, but, as illustrated above, in ERα -positive breast cancers, there are many ERα -negative cells mixed in with the ERα -positive (brown stain) cells. This image is an example of an invasive ductal cancer of the breast stained with ERα -specific antibody 1D5 (Dako). Cells are counterstained with hematoxylin.
ERβ expression. (Inset) ERβ is widely expressed in breast cancer, but the clinical significance of this is not clear. This image is an example of invasive ductal cancer of the breast stained with an ERβ -specific polyclonal antibody (Upstate Biotech).
- © American Society for Pharmacology and Experimental Theraputics 2002
References

Otabek Imamov, MD, received his training in urology in Russia and is, at present, a Graduate Student at the Department of Medical Nutrition, Karolinska Institute, Novum, Huddinge, Sweden.
Yoko Omoto, MD, PhD, trained in Japan with an interest in hormone-related cancers. She is presently a postdoctoral fellow at the Department of Medical Nutrition, Karolinska Institute, Novum, Huddinge, Sweden.
Margaret Warner, PhD, received her training in pharmacology at McGill University. She is presently a Research Scientist at the Department of Bioscience, Karolinska Institute, Novum, Huddinge, Sweden.
Guojun Cheng, MD, PhD, received his training in gynecology in China, and is a postdoctoral fellow in the Department of Medical Nutrition, Karolinska Institute, Novum, Huddinge, Sweden.

Jan-Åke Gustafsson, MD, PhD, received his training in biochemistry at the Karolinska
Institute. He is presently a Professor of Medical Nutrition and Director of Center for BioTechnology, Karolinska Institute, Novum, Huddinge, Sweden. Please address correspondence to J-ÅG. Email jan-ake.gustafsson{at}mednut.ki.se