
- Institution: Stanford Univ Med Ctr Lane Med Lib/Periodical Dept/Rm L109
- Sign In as Member / Individual
Targeting the FLICE Inhibitory Protein (FLIP) in Cancer Therapy
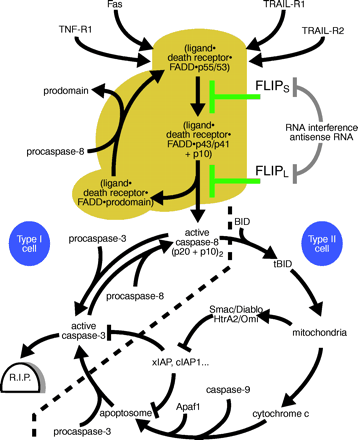
Apoptotic signaling pathways induced by death receptors and their regulation by FLIP.
In type I cells, death receptors (e.g., Fas) induce strong caspase-8 activation, which alone is sufficient to lead to robust processing of effector caspases such as caspase-3 and apoptosis induction (events shown left of the bold dashed line, golden brown region). In type II cells, only low amounts of caspase-8 are activated upon DISC formation. In addition, the action of effector caspases processed by caspase-8 is blocked by IAP proteins. Apoptosis induction in this type of cells is therefore dependent on a mitochondrial amplification loop, which triggers a second pathway leading to caspase-3 activation and furthermore interferes with the action of IAP proteins. IAP, inhibitor of apoptosis protein; BID, BH3-interacting domain death agonist; tBID, truncated BID (a caspase-derived cleavage product of BID); TRAIL, tumor necrosis factor–related apoptosis-inducing ligand; SMAC, second mitochondria-derived activator of caspase; Diablo, direct IAP binding protein with low pI; HtrA2, human serine protease with significant similarity to E. coli HtrA (HtrA2 is also called Omi); Apaf1, apoptotic protease activating factor 1.


