S-Nitrosylation Signaling in Cell Biology
Abstract
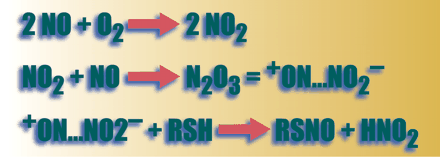
S-Nitrosylated proteins form when a cysteine thiol reacts with nitric oxide (NO) in the presence of an electron acceptor to form an S-NO bond. Under physiological conditions, this posttranslational modification affects the function a wide array of cell proteins, ranging from ion channels to nuclear regulatory proteins. Recent evidence suggests that 1) S-nitrosylated proteins can be synthesized by exposure of specific redox-active motifs to NO, through transnitrosation/transfer reactions, or through metalloprotein-catalyzed reactions; 2) S-nitrosothiols can be sequestered in membranes, lipophilic protein folds, or in vesicles to preserve their activity; and 3) S-nitrosothiols can be degraded by a number of enzymes systems. These recent insights regarding the bioactivities, molecular signaling pathways, and metabolism of endogenous S-nitrosothiols have suggested several new therapies for disease ranging from cystic fibrosis to pulmonary hypertension.
- © American Society for Pharmacology and Experimental Theraputics 2003



