
- Institution: Stanford Univ Med Ctr Lane Med Lib/Periodical Dept/Rm L109
- Sign In as Member / Individual
PLC-ε: A Shared Effector Protein in Ras-, Rho-, and Gαβγ-Mediated Signaling
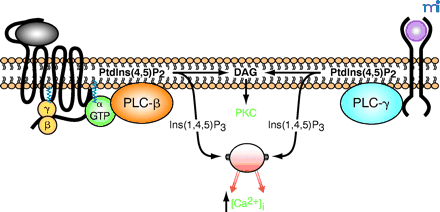
Receptor-promoted activation of phospholipase C and generation of second messengers. A prototypical G protein–coupled receptor is schematized as a black string (left) with seven transmembrane domains. Binding of a hormone ligand (gray oval) results in activation and dissociation of heterotrimeric G proteins into free Gβγ and GTP-bound α subunits. In accordance with the historical view, the GTP-bound α subunit activates an isoform of PLC-β, which hydrolyzes phosphatidylinositol 4,5-bisphosphate [PtdIns(4,5)P2] into inositol 1,4,5-trisphosphate [Ins(1,4,5)P3], which raises the cytosolic concentration of calcium ions ([Ca2+]i) and diacylglycerol (DAG), an activator of protein kinase C (PKC). Also schematized is a receptor tyrosine kinase (right) bound by an extracellular growth factor (pink circle); the epidermal growth factor receptor (EGFR) conforms to this schematization. Traditionally, this second class of receptor was proposed to initiate an inositol lipid response through activation of an isoform of PLC-γ as a consequence of tyrosine phosphorylation of the isozyme.


