
- Institution: Stanford Univ Med Ctr Lane Med Lib/Periodical Dept/Rm L109
- Sign In as Member / Individual
CaMKII, an Enzyme on the Move: Regulation of Temporospatial Localization
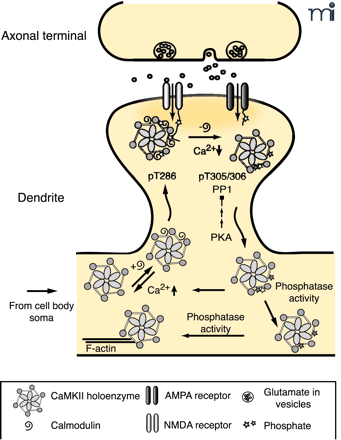
The role of autophosphorylation in dynamic activity-dependent translocation of CaMKII to the PSD. Synaptic activity leads to NMDA receptor activation and Ca2+ influx allowing Ca2+-CaM to bind to CaMKII promoting translocation to the PSD. Autophosphorylation at T286 (bright star) traps CaMKII in the PSD until Ca2+ levels decrease and CaM dissociates from CaMKII. CaMKII autophosphorylated at T305, 306 (dark star) is unable to rebind CaM, leading to dissociation of CaMKII from the PSD. PP1 activity, which can be overcome by increased PKA activity, dephosphorylates pT286 and facilitates the dissociation process. The cytosolic pool of CaMKII exists as a mixed population of kinases, with respect to their different states of autophosphorylation. Those with residual pT286 are primed to translocate to the PSD rapidly with a lower amplitude synaptic stimulus, whereas kinase with pT305/306 requires cytosolic phosphatase activity to return to the basal state (19, 44, 71).


