Serotonin Transporter: Gene, Genetic Disorders, and Pharmacogenetics
- 1Laboratory of Clinical Science, Building 10, Room 3D41, 10 Center Drive, MSC 1264, NIMH, NIH, Bethesda, MD 20892;
- 2Department of Pharmacology, Yale University School of Medicine, New Haven, CT 06510;
- 3Molecular and Clinical Psychobiology, Department of Psychiatry and Psychotherapy, University of Würzburg, Würzburg, Germany
- Please address all correspondence to DLM. Email murphyd{at}intra.nimh.nih.gov; fax 301-402-0188.
Abstract
The highly evolutionarily conserved serotonin transporter (SERT) regulates the entire serotoninergic system and its receptors via mod-ulation of extracellular fluid serotonin concentrations. Differences in SERT expression and function produced by three SERT genes and their variants show associations with multiple human disorders. Screens of DNA from patients with autism, ADHD, bipolar disorder, and Tourette’s syndrome have detected signals in the chromosome 17q region where SERT is locat-ed. Parallel investigations of SERT knockout mice have uncovered multiple phenotypes that identify SERT as a candidate gene for additional human disorders ranging from irritable bowel syndrome to obesity. Replicated studies have demonstrated that the SERT 5′-flanking region polymorphism SS genotype is associated with poorer therapeutic responses and more frequent serious side effects during treatment with antidepressant SERT antagonists, namely, the serotonin reuptake inhibitors (SRIs).
Introduction
A recent explosion of molecular genetic studies and clinical findings has brought the serotonin (also termed 5-HT) transporter into the spotlight. Since the human serotonin transporter gene (SLC6A4, SERT, 5HTT) was sequenced and cloned a decade ago, SERT homologs have been identified in over ten species (Figure 1⇓), setting the scene for comparative investigations of SERT molecular genetics and function in non-human primates, rodents, worm (C. elegans), fly (Drosophila), and even in prokaryotes (1, 2). Moderately high sequence homology (~50%) with SERT is also present for the dopamine transporter (DAT) (encoded by SLC6A3) and the norepinephrine transporter (NET) (encoded by SLC6A2) which, along with transporters for glycine and γ-aminobutyric acid (GABA), belong to the family designated the neurotransmitter:sodium symporter (NSS) family 2.A.22 (3, http://www-biology.ucsd.edu/msaier/transport/). The large-solute carrier (SLC) gene the forty-three families [Human Genome Organization (HUGO) - superfamily now includes a total of 298 members categorized into Web site, http://www.gene.ucl.ac.uk/hugo/]. Genetic disruptions of murine SERT (mSERT) and of some murine genes coding for the 5-HT receptors whose activities are affected by SERT alterations have led to new insights into the complexity of the serotonin neurotransmitter system (4–7).
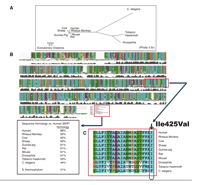
Evolutionary relatedness and sequence comparison of SERT from several species. A. Phylogenetic tree for SERT, showing relative evolutionary distances among species for SERT protein sequences, constructed using Phylip 3.5c and 3.6 (beta version as of January 2004) (J. Felsenstein, 1993, Department of Genetics, University of Washington, Seattle, WA; http://evolution.genetics.washington.edu/phylip.html). B. Specific conservation of SERT sequences relative to human SERT with >90% homology in vertebrates and lesser homology in insects and worm (44–53%). C. Enlarged segment of SERT TM8 region illustrating high conservation of the transmembrane region where the I425V missense mutation is located (see Box 1).
These new insights have occurred against a backdrop of twenty years of investigations of therapeutic effects of the serotonin reuptake inhibitors (SRIs), drugs whose primary target is SERT. These SERT antagonists are the single most frequently prescribed group of psychoactive drugs that are used in the treatment of depression, obsessive–compulsive disorder, and anxiety disorders as well as other conditions such as migraine, chronic pain, and alcoholism. Another basis for sustained high interest in SERT is that it is a major target site for such drugs of abuse as MDMA (“ecstasy”) and cocaine. Verification of SERT’s importance for drugs of abuse comes from studies of mice with reduced or absent mSERT following targeted mutagenesis. These mice have reduced or absent locomotor responses to MDMA and have enhanced reward responses to cocaine (4, 8). On the other hand, new experimental epistatic data indicate that mSERT loss together with mDAT loss results in absent cocaine reward behavior, where-as mSERT loss together with mNET loss confers greatly enhanced cocaine reward behaviors (9, 10).
This report surveys recent developments from investigations of SERT in multiple species. A special focus is on how molecular and genetic studies of SERT may inform quantitative trait investigations of SERT variants in disease susceptibility and health enhancement, including pharmacological and gene-based treatment of disease.
Serotonin Transporter Gene, Variants, and Function
SERT in brain and in many peripheral tissues is responsible for the active transport of serotonin into neurons, enterochromaffin cells, platelets, and other cells. In brain, SERT is situated both in perisynaptic membranes of nerve terminals and in dendritic arbors in close proximity to serotonin-containing cell bodies in the midbrain and brain stem raphe nuclei. SERT mediates rapid removal and recycling of released serotonin following neuronal stimulation. Thus, it has a critical role in the homeostatic regulation of the magnitude, duration, and spatial distribution of signals reaching serotonin receptors. SERT has some ability to transport other endogenous amines such as dopamine, and also is a drug transporter for agents that include fenfluramine, nonfenfluramine, substituted amphetamines (i.e., MDMA, and parachloroamphetamine), methcathinone, and the serotonin (but not dopamine) neurotoxin amino-MPTP [1-methyl-4-(2′-aminophenyl)-1,2,3,6-tetrahydropyridine], which enters neurons via SERT to induce persistent (months-long) reductions of serotonin accompanied by neurodegenerative changes (11–14).
At the structural level, SERT’s function as a transporter protein in membranes remains incompletely understood. SERT and other Na+- and Cl−-dependent NSS solute carriers for monoamines as well as the amino acid transporters for glycine, GABA, and other substrates are predicted to contain twelve hydrophobic transmembrane (TM) domains. SERT is thought to operate primarily by an alternating access mechanism, in which a single binding site, accessible from the cell exterior, binds Na+, Cl−, and 5-HT simultaneously. When the binding site is full, a conformational change is triggered that closes the site from access to the external medium and opens access to the cytoplasmic surface of the membrane. After the dissociation of 5-HT, Na+, and Cl−, K+ binds to the same binding site to facilitate the conformational change back to the form with an extracellularly accessible binding site. In this process, a single multifunctional binding site is responsible for movement of Na+, Cl−, 5HT, and Transport of all of these SERT substrates depends upon maintenance of ion gradients across the cell membrane by Na+-K+– ATPases.
Increasing quaternary structure evidence suggests that SERT molecules operate as dimers or higher-ordered oligomers, specifically tetramers (15), consistent with data regarding DAT and GAT-1 (16, 17). In the absence of sufficient quantities of solubilized membrane proteins for even initial studies, the only available structural 3-D models for transporters like SERT are bacterial transporters such as lactose permease (18), the glycerol-3-phosphate transporter (19), and the adenine nucleotide exchanger in mitochondria (20). These models raise the possibility that the twelve-TM-containing transporter proteins may move substrates through an interface between subunits or between domains within a subunit. Nevertheless, in the absence of any direct supporting data, such extrapolations require caution.
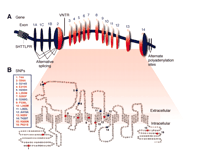
Human SERT spans 37.8 kb on chromosome 17q11.2, and is composed of fourteen exons encoding a protein of 630 amino acids (Figure 2⇓) (21, 22). Alternate promoters in combination with differential splicing involving exon 1A, B, and C in specific tissues, and alternate polyadenylation site usage resulting in multiple mRNA species are likely participants in the regulation of SERT expression in humans (Figure 2⇓) (23, 24). In addition to several regulatory domains controlling selective expression in serotoninergic neurons, transcriptional activity of human SERT is modulated by a repetitive element of varying length in the 5′ flanking region located ~1.4kb upstream of the transcription start site, termed the 5HTT gene-linked polymorphic region (5HTTLPR). Three additional variations have been described in untranslated regions: 1) a ~380bp deletion [del(17)(q11.2)] between the 5HTTLPR and the transcription start site which may be unstable in vivo, conferring mosaicism with cell or tissue differences in expression of SERT, and possibly involving 5HTTLPR-dependent mechanisms (25–27); 2) a variable number tandem repeat (VNTR) within intron 2; and 3) a G to T transversion in a putative adenylation site in the 3′ UTR region (28, 29).
Organization of SERT and location of polymorphisms. A. SERT gene organization showing coding (orange) exons and non-coding and intronic areas (blue), locations of the 5HTTLPR polymorphism ~1.4 kb upstream and location of the intronic VNTR near exon 2. B. Translated (red) and non-translated (blue) SNP sites are annotated in the 630 amino acids of the 12-TM-containing SERT protein (54, 157, 158).
The 5HTTLPR is only present in humans and higher non-human primates; rhesus monkeys have an analogous 5′ flanking region length variant generated by a 21bp insertion or deletion (rh5HTTLPR), whereas prosimians and rodents lack such length variants (30). Murine SERT, located at chromosome 11.42, possesses transcriptional control elements highly similar to the human gene, including cAMP-dependent components plus some evidence for alternative exon 1 usage in different tissues (23, 31–33).
5HTTLPR alleles are most commonly composed of either fourteen (“short” or “S” allele) or sixteen (“long or “L” allele) repeated elements (each of 20 to 23 bp), although alleles with more repeat elements (“superlong”, XL and XXL, with up to twenty repeats) occasionally occur, as do variants with single-base insertions, deletions, or substitutions within individual repeat elements (34, 35). The uncommon, super-long alleles arose from duplication of an internal segment of 5HTTLPR composed of repeat elements VI to IX, including the specific elements deleted in the S allele (36). A predominantly North American/European population displayed allele frequencies of 57% for the L allele and 43% for the S allele with a 5HTTLPR genotype distribution of 32% LL, 49% LS, and 19% SS (37); different allele and genotype distributions are found in other populations, particularly Asians who have over two-fold higher SS genotype frequencies (36, 38). The S and L 5HTTLPR variants differentially modulate transcriptional activity of the SERT promoter, yielding differences in SERT mRNA and protein density, and 5HT uptake activity in human lymphoblastoid cells, platelets, and brain (37, 39, 40). That the variant is associated with lower SERT expression and function has been confirmed by studies of hSERT promoter activity in other cell lines (26), mRNA concentrations in the raphe complex of human postmortem brain (40), platelet 5HT uptake and content 39, 41), 5HT system responsivity elicited by pharmacologic challenge tests with clomipramine and fenfluramine (42, 43), intracortical inhibition responses to citalopram (44), and mood changes following tryptophan depletion (45).
The intron 2 VNTR contains nine, ten, or twelve copies of a sixteen- or seventeen–base pair repeat (46). Data indicative of its functionality include stronger expression by the twelve-copy allele than by the ten-copy allele in hindbrain of transgenic embryonic mice and greater enhancer activity in embryonic stem cells, although not in HeLa cells (5, 47, 48). These effects are dependent upon individual repeat elements within the VNTR domain, and are tissue-specific, suggesting that studies of the association of this VNTR with human disorders need to take into account not only copy number but also interactions within the primary sequence of the repeat unit (48). Increasing evidence suggests that a similar complexity of transcriptional regulation may also be found within the SERT 5′ flanking region including the 5HTTLPR and also in regulatory-region length variants of other genes such as the transcriptional control region variants in the insulin-dependent diabetes mellitus 2 (IDDM2) gene (49, 50), and the 5-HT1B receptor gene (51). Another source of complexity within SERT apparently results from recombination events in the vicinity of exon 1, leaving the intron 2 VNTR in good linkage dis-equilibrium (LD) with SNPs 3′ to this VNTR, but with very little significant LD between 5HTTLPR and the intron 2 VNTR together with all of the SNPs 3′ to the intron 2 VNTR (52). (Linkage equilibrium exists when alleles at two distinctive loci occur more frequently than expected given the known allele frequencies and the recombination fraction between the two loci. Recombination fraction is the frequency of crossing over between two loci.) One consequence of these observations is that >90% of investigations of SERT as a candidate gene for neuropsychiatric or other disorders have only genotyped 5HTTLPR; specific involvement of most of SERT has remained unexamined. Remarkably, a similar situation exists for DAT, namely that negligible LD exists between two main segments of DAT divided by a partitioning between exon 6 and 9 (53). At least twenty-five additional SNP variants are found in hSERT locus, eight of which are regarded validated, as of January 2004 (NCBI SNP website) (Figure 2⇑). Among fifteen SERT SNPs whose frequency was evaluated in 450 individuals from the general US population, most are uncommon or very rare, and are equally divided into synonymous and non-synonymous SNPs (54). (Synonymous SNPs are nucleotide codons, which code for same amino acid.) The capacity of most of the non-synonymous variants to alter SERT function, and their potential involvement in disease generally remains to be explored. Two exceptions are I425V and L255M, which are non-synonymous rare SERT missense mutations in highly conserved transmembrane regions (Figure 1⇑) that were found to segregate with complex serotoninergic dysfunction-related phenotypes including obsessive–compulsive disorder in the case of I425V (55–57), or to accompany severe depression in the case of L255M (58) (Box 1).
Rare SERT Variants and Neuropsychiatric Disorders.
Obsessive-Compulsive Disorder (OCD): A missense mutation resulting in the conservative I425V substitution in SERT was recently detected in two unrelated families with OCD and related disorders (55). Six of seven family members with the mutation had OCD or OC personality disorder while none of the seven other family members were affected. In addition, the affected individuals and their immediate family members carrying the I425V variant met diagnostic criteria for other disorders including Asperger’s syndrome (an autism variant), social phobia, anorexia nervosa, tic disorder, depression, and alcohol abuse/dependence.
I425V is evolutionarily conserved (Figure 1⇑) and, except for the seven individuals in these two families, I425V has otherwise only been found in one anonymous individual of 450 in an unscreened general population sample (54). I425V is located in TM8 and may modify the αhelical secondary structure of SERT and consequently transport function. In fact, expression studies of the mutant SERT cDNA in human cells demonstrated a gain-of-function via constitutive activation of serotonin transport through a process also stimulated by nitric oxide and cyclic GMP, resulting in a ~2-fold increase in serotonin uptake (56).
Interestingly, two brothers and two sisters from the two families suffering from OCD and autism or anorexia nervosa and carrying I425V also had the LL 5HTTLPR genotype which was previously found in some (122, 135, 148, 149) but not all (150) studies to be associated with or preferentially transmitted in both OCD (122, 135) and autism (148, 149). Thus, since both I425V and LL 5HTTLPR lead to a gain of expression and function, additive, or interactive consequences––most likely during neurode-velopment––might be involved in this rare situation.
Severe Depression: A second conservative substitution, located in SERT TM4 L255M, was detected in a patient with delusional depression, who was also found to carry the 5HTTLPR SS genotype that is implicated in anxiety- and depression-related traits (58). Possibly, low SERT gene expression in interaction with the L255M variant, at a locus that is highly conserved across multiple species (Figure 1⇑), could additively perturb SERT function or regulation.
Perspectives: These two examples of co-occurrence and possible cooperativity of allelic variation in gene expression and protein structure might represent “double-hits” with functional consequences in the same gain- or loss-of-function direction for these SERT gene variations. This notion requires further functional analysis, such as evaluations of ion transport or substrate characteristics, or response to drugs that affect serotoninergic signaling including SRIs. Among the other monoamine transporters, the only analogous finding of a rare variant associated with disease is the hNET A457P variant, which segregated in one family with a syndrome of orthostatic intolerance including postural heart rate and catecholamine abnormalities (151); this variant results in a loss of function related in part to diminished membrane surface expression of hNET as well as to reduced affinity for its substrate, norepinephrine (152, 153). This A457P hNET variant, like the I425V SERT variant, are the first examples of highly penetrant alleles with striking familial aggregations of disorders found for the monoamine transporters. Thus, they resemble the rare vari-ants accounting for genetic familial subtypes of common disorders such as α-synuclein for Parkinson Disease, APP, and presenilin 1 and 2 for Alzheimer Disease (154, 155) and MEF2A for coronary artery disease (156).
SERT Localization and Brain Imaging of hSERT Using PET and SPECT
SERT mRNA in brain closely parallels the distribution of tryptophan hydroxylase in serotonin-containing cell bodies, with concentrations highest in the dorsal raphe, lower concentrations in other raphe nuclei and low concentrations in a few other areas such as the dorsomedial nucleus of the hypothalamus and cortex (59). Likewise, SERT protein expression is highest overall in raphe areas; during development, it extends in spatiotemporal congruence with axons of raphe neurons, prior to synapse formation. An exception is the transient expression of SERT protein and mRNA during early postnatal development in two patterns: 1) together with vesicular transporter (VMAT2) mRNA in trigeminal, cochlear and solitary nuclei of the brainstem and sensory nuclei of the thalamus (ventrobasal, lateral and medial geniculate); and 2) without VMAT2 mRNA in some limbic areas including septum, olfactory areas, and hippocampus (60).
Most human brain imaging studies of SERT in the past decade used [123I]-βCIT [(I-2β-carbomethoxy-3β-(4-iodophenyl)tropane] (RTI-55) in SPECT studies, although βCIT’s equal affinity for DAT results in visualization of SERT verified by SRI antagonism only in a single midbrain region. There is evident need for replication of the results found with βCIT using positron emission tomography (PET) methodology. In fact, more selective SERT ligands have become available recently for PET studies including McN 5652 ([+]-6β-(4-methylthiophenyl)-1, 2, 3, 5, 6α, 10β-hexahydropyrrolo[2,1-a]isoquinoline), and a group of diarylsulfides, namely DASB (3-amino-4[2-(dimethyl-aminomethylphenylthio)] benzonitrile), AFM (2-[2-(dimethyl-aminomethylphenylthio)]-5-fluoromethylphenylamine), ADAM (2-[2-(dimethylaminomethylphenylthio)]-5-iodophenylamine, and MADAM (2-[2-dimethylaminomethyl-phenylsulphanyl]-5-methyl-phenylamine) (61). The latter three compounds have not yet been evaluated in humans, but have promising properties. McN 5652 has fair specificity for SERT, although high non-specific binding prohibits visualization of important human brain areas that have lower SERT density such as cortex and limbic system. Another disadvantage is that McN 5652 exhibits very slow kinetics, such that within a typical imaging time of ninety minutes, one can only measure the rate of brain uptake of this ligand with virtually no washout measurement possible. Without measurement of both uptake and washout, the affinity for the receptor is difficult to quantitate (61).
DASB, radiolabeled with 11C, is poised to become the most widely used PET ligand to visualize SERT binding sites in human brain. DASB is highly selective for cloned hSERT, with nanomolar affinity for SERT (Ki = 1nM) and much lower, micromolar affinity for the dopamine transporter (DAT), (Ki = 1420 nM), and nor-epinephrine transporter (NET), (Ki = 1350 nM) (62). DASB penetrates the blood–brain barrier well and is rapidly metabolized, such that approximately 50% of the parent compound is cleared from plasma by twenty minutes post-injection (63, 64). The metabolites of DASB are mainly polar, hydrophilic species, which are unlikely to pass the blood-brain barrier. Scanning time required for DASB is the shortest of all for the newly available ligands (61).
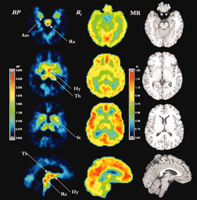
PET studies in humans show that [11C]-DASB binds reversibly to SERT throughout the brain. The highest uptake is in hypothalamus; intermediate levels are seen in the midbrain, thalamus, and striatum with lower levels observed in cortical regions and white matter and with no apparent binding in the cerebellum (63–65) (Figure 3⇓). These data agree with SERT densi-ties found in post-mortem human brain (66, 67) and in rodent brain regions (31, 63). A simplified method of [11C]-DASB tissue data analysis using a one-compartment model has been proposed for routine use where the cerebellum, because of its negligible levels of SERT binding, is used as a reference tissue to estimate SERT binding poten-tial instead of an arterial input function (63, 64). [11C]-DASB displays quite high signal-to-noise ratios in vivo (63, 65).
Using imaging techniques to identify the location of SERT. Positron emission tomography of SERT, showing parametric images of [11C]-DASB binding potential (BP) (left column) and relative delivery (R1) (middle column) estimated by the two-parameter multilinear reference tissue model, MRTM2. MRTM2 is a data analysis method that allows “volume pixel” (i.e., “voxel”)-wise mapping of SERT density (BP) and relative blood flow (R1) from dynamic [11C]-DASB PET data. (Voxelization is a process whereby the addition of depth to an image is gained by using a set of cross-sectional images.) Magnetic resonance (MR) images (right column) are shown for comparison. Upper rows are transverse images and the bottom row presents sagittal images. The color display ranges for BP images are 0 to 3.0 and for R1 images are 0 to 1.50 with higher numbers indicating greater SERT density (BP) or greater relative blood flow (R1) reveal where SERT may be more highly concentrated in brain areas. Am, amygdala; Hy, hypothalamus; Ra, raphe complex; St, striatum; and Th, thalamus. This figure was kindly provided by Drs. M. Ichise and R. Innis (64).
[11C]-DASB’s specificity for SERT in vivo in humans was demonstrated by an 80% decrease in brain radioactivity after blockade of SERT sites with a single 40 mg oral dose of the highly selec-tive SERT inhibitor, citalopram (65). In addition, treatment of patients with depression using lower doses (20 mg/day) of either paroxetine or citalo-pram for four weeks produced a mean decrease of 80% in striatal [11C]-DASB-labeled SERT sites (68). In mice with a targeted disruption of SERT studied autoradiographically with []-DASB, SERT−/− mice had no specific binding, whereas SERT+/– mice had a ~50% decrease in SERT sites in all brain regions, consistent with prior studies using the ligands RTI-55 and cyanoimipramine in these mice, and thus validating DASB as a highly specific SERT ligand (31, 69, 70).
Disorders with Implicated SERT Involvement
SERT may contribute to several human disorders, based on the genetic association studies focused primarily on the 5HTTLPR polymorphism, and to a lesser degree on the intronic VNTR near exon 2. A few recent studies have begun to incorpo-rate SERT region SNPs and haplotypes (71–73). Simple case-control comparisons have been most common, and most reports (>300 total) comprise investigations of neuropsychiatric phenotypes, some of which have been par-tially catalogued and reviewed elsewhere (54, 74, 75). Many of these studies acknowledge limitations, which often include small sample sizes, recognized, or suspected variability in the clinical phenotype, and coexisting disorders. Nonetheless, a few examples of special interest warrant discussion, including several examples of apparent SERT-related dysfunction in the periphery, a somewhat neglected area of interest (76).
Affective Disorders: Major Depressive Disorder, Recurrent Depression, Bipolar Disorder, and Suicide
The different affective disorders, plus a separate complex behavior, suicide, have been the object of many association studies with SERT, as well as several genome-wide scans––that is, testing the chosen trait for linkage to polymorphic markers spread through-out the genome. The latter have been principally focused on bipolar disorder and recurrent affective disorder (74). Although reliable diagnoses can be made of these disorders based on strong rater agreement, boundary problems arise because of overlapping phenotypes, including some extending towards psychosis (e.g., schizoaffective disorder, psychotic depression) as well as with other “primary” neuropsychiatric disorders such as obsessive-compulsive disorder (OCD). For example, probands ascertained as bipolar have ~20% coexisting OCD, whereas studies of individuals with a primary diagnosis of OCD reveal a ~10% incidence of comorbid bipolar disorder (77).
In addition to phenotypic diversity, evidence of genetic heterogeneity has been commented on in several studies of bipolar disorder (78). Gene–environment interactions are another source of complexity. An interesting recent study––one of the very few to tackle this latter question epidemiologically in humans––evaluated >800 individuals followed since age three. The hypothesized interaction of the 5HTTLPR genotypes and of recent traumatic life events upon depression diagnosis and other depression-related problems like suicide was confirmed: more depressive events occurred in individuals who had the SS genotype and who had four or more recent life traumas, whereas those with the LL geno-type were protected (79). One interpretation suggested the possibility that 5HTTLPR may not directly associate with depression, but may rather modulate the serotoninergic response to stress; this interpretation was derived from 1) recent brain imaging findings of exaggerated amygdala responses to fearful stimuli in individuals with the S allele and 2) exaggerated ACTH and epinephrine and other neuroendocrine responses to handling or restraint in transgenic mice with reduced or absent SERT (7, 79–81).
The dependence of a strong association of depression with SERT upon the environmental influence of severe life-events illustrates a principle known from murine genetic models, including specific studies of SERT-stress interactions (7, 81), namely that gene effects in complex human disorders may only be identifiable and prove etiologically important when coupled with additional factors. Similarly, specific synergism, antagonism, or more complex, quantitative alterations have been observed for monoamine oxidase (MAO), brain-derived neurotrophic factor (BDNF) and three other genes in model epistatic investigations of mice with reduced or absent SERT expression (82). As mentioned above and reviewed in Box 1, interactions among two or more SERT variants may also yield more extreme clinical phenotypes (55, 56).
Primary Pulmonary Hypertension
The progressive and frequently fatal disorder primary pulmonary hypertension (PPH) is characterized by blood pressure increases associated with abnormal vascular proliferation in the pulmonary artery bed. Serotonin is a potent inducer of smooth muscle cell proliferation in lung vessels, an effect that is attributable to serotonin’s internalization by SERT as it is dose-dependently inhibited by SRIs but not by serotonin receptor antagonists (83). Iatrogenic pulmonary hypertension has been identified during the last decade as the result of treatment of obesity by fenfluramine alone or together with phentermine (84, 85). Fenfluramine is a SERT substrate that also releases serotonin from vesicles once it enters cells via SERT (13). Individuals with spontaneous PPH more frequently have the 5HTTLPR LL genotype together with greater SERT mRNA and immunoreactivity in lung tissue as well as greater uptake of serotonin in platelets (83). Hypoxia can lead to smooth muscle hyperplasia. Hypoxia leads to increased expression of SERT in lung vessels; a causative role for hypoxia in pulmonary hypertension has been inferred from evidence that mice lacking SERT are protected against hypoxia-induced pulmonary hypertension (86, 87). Unlike PPH patients, hypoxic patients with chronic obstructive pulmonary disease (COPD) do not differ from controls in 5HTTLPR genotype frequencies; however, the LL variant of 5HTTLPR in COPD patients, who have higher SERT expression in pulmonary artery cells, had significantly more severe pulmonary hypertension than did LS or SS patients, a finding which is in accord with findings in rodent models (87, 88). In addition, a complementary, epistatic interaction has been proposed between this 5HTTLPR genotype and a common bone morphogenetic protein 2 receptor (BMPR2) variant, which is also involved in smooth muscle cell proliferation and has additionally been implicated in familial PPH (89). These results from both humans and rodents raise the question of whether SRIs might constitute an experimental treatment for PPH.
Myocardial Infarction
Two studies, which together enrolled 976 individuals with myocardial infarction (MI) and 926 controls, found significantly greater risk for MI in individuals with the LL genotype (90, 91). Remarkable complementary evidence from a study of over 5,000 individuals including >1000 with MI found significant protection (an odds ratio of 0.59) from MI in individuals receiving antide-pressants with high SERT affinity (92). Furthermore, increased SERT affinity among the different SRIs was correlated with reduced risk of MI in these SRI-treated patients (92). Potential interactions with smoking history, platelet aggregation, plasma lipids including cholesterol, depression, anxiety and other variables like cardiovascular reactivity may be involved (90, 91, 93–95).
Irritable Bowel Syndrome and its Treatment
Gut tissue has, by far, the greatest (95%) amount of serotonin in the body. Serotonin participates in the local regulation of gut function. Its release from mucosal enterocytes initiates peristaltic and secretory reflexes, whereas its release from enteric neurons mediates fast and slow excitatory neurotransmission. SERT helps to regulate the activity of serotonin in the bowel via SERT’s presence in several cell types, including crypt and villus epithelial cells (enterocytes) and enteric serotoninergic neurons. Both in the mucosa and in the enteric nervous system, SERT terminates serotonin’s actions (96).
In one case-control study of IBS, the SS genotype was significantly more frequent in a diarrhea-predominant subgroup, although no overall genotype difference from controls was found (97). IBS treatment responses (reductions in colonic transit time) to the 5-HT3 receptor antagonist, alosetron, were significantly more prevalent in diarrhea-predominant patients with the LL genotype (98). Of related interest, mice lacking SERT have increased gut motility together with frequent diarrhea alternating with occasional constipation in an IBS-like fashion (99). Adaptive changes occur in the subunit composition of enteric 5HT3 receptors of SERT−/− mice. Expression of the 5HT3B subunit is decreased and this is accompanied by a decrease in the affinity of 5HT3 receptors for 5HT and a greater tendency of 5HT3 receptors to desensitized. These changes are protective against an environment of excessive 5HT availability (100).
Other Studies
Linkage has been observed to 17q regions containing SLC6A4, the SERT locus in genome-wide scans of DNA collected from individuals with various disorders including bipolar disorder (101–103); ADHD (104, 105); Tourette’s syndrome (106), and autism (107, 108).
In addition, investigations are being conducted of potential disease-related quantitative traits––also termed endophenotypes, which include biochemical, endocrinological, or other physiological and psychological measurements––studied in human populations that may interact with other vulnerability factors, sometimes with gender and ethnicity differences (73) that contribute to specific disorders. Some examples include personality characteristics such as anxiety, neuroticism, and harm avoidance (37, 109), fear conditioning (110), and exaggerated amygdala or cardiovascular responsivity to stressful and other stimuli (80, 94, 111).
Furthermore, results from investigations of mice with reduced or absent serotonin transporters suggest that SERT differences may contribute to additional genetically complex disorders including polysubstance abuse (1, 4, 9, 112), REM sleep disorders (113), obesity (114), gastro-intestinal disorders in addition to IBS (99), pain-related disorders (115), anxiety disorders (6, 7, 82, 116–118). A general principle underlying all of these proposals of SERT as a vulnerability factor for multiple CNS and peripheral disorders is that genetic variants of SERT may act both in the brain and in specific organs like heart, lung, gut, and adrenal plus other endocrine glands during development and adulthood (119, 120).
A Role for SERT in SRI Pharmacogenetics, Therapeutic Responses, and Major Side Effects
The serotonin reuptake inhibitors (SRIs) include fluoxetine, fluvoxamine, paroxetine, sertraline, citalopram and escitalopram, all of which are highly potent (Ki ~1 nM) and selective inhibitors of SERT’s function at presynaptic terminals, axons, dendrites, neuronal cell bodies and other cells that express SERT. Blockade of SERT by SRIs under normal circumstances has minimal immediate consequences for physiological or behavioral endpoints in humans. Repeated, long-term administration for three-to-six weeks is required for antidepressant actions and most other beneficial effects, while treatment for ten-to-twelve weeks or longer is required for efficacy in obsessive–compulsive disorder, as slowly-developing adaptation in receptor and post-receptor neuronal signaling, neurotrophic responses and plastic remodeling of brain regions including hippocampus appears to be required (121).
Among the other large class of less-selective reuptake-inhibiting tricyclic antidepressants, clomipramine, has relatively greater efficacy at SERT than does desipramine or the other tricyclics. Although depression and most anxiety disorders respond equally well to all tricyclics, SRIs, selective norepinephrine transporter inhibitors, and selective serotonin-norepinephrine combination uptake inhibitors, treatment efficacy in OCD and autism is restricted to the SRIs or to clomipramine (122, 123).
SRI Drug Metabolism
Most investigations of the role of genes in individual differences in drug response have focused on pathways involved in drug metabolism (124). All tricyclic antidepressants and most SRIs (fluoxetine, fluvoxamine, paroxetine, and desmethylcitalopram, but not citalopram itself) are substrates of the drug-metabolizing enzyme, CYP2D6; some of these and other SRIs are metabolized by CYP3A4 (e.g., sertraline and norfluoxetine) or CYP1A2 (fluvoxamine). Variants in the genes that encode these enzymes occur and can result in ultrarapid or slow metabolism of these drugs in 7–10% of Caucasians, with resultant drug plasma level differences in patients (125).
Drug-metabolizing variability among patients is of special clinical concern because tricyclic antidepressants have a relatively narrow therapeutic index and exhibit significantly increased toxicity at elevated drug concentrations. In contrast, SRIs have a flat dose-response curve and wide therapeutic index, and hence toxicity due to excessive drug concentrations seems to be uncommon. However, the 1–10% of Caucasians who are CYP2D6 specific ultra-rapid metabolizers would seem to be at some risk for SRI under-dosing (125, 126). In addition, both SRI and tricyclic drug metabolism may be affected by drug–drug interactions due to the large number of agents that are substrates of these hepatic enzymes. Of course, many independent non-gene factors including gender, age, body fat, disease, and time of administration as well as ethnic differences may affect drug concentrations in plasma and tissues, including brain.
SERTand SRI Pharmacogenetics
Associations between the SERT 5HTTLPR polymorphism and both therapeutic responses and adverse reactions to SRIs have been reported. Seven out of nine investigations, which enrolled a total of 877 individuals, found the LL 5HTTLPR genotype or L allele predictive of better antidepressant efficacy in studies of fluvoxamine, fluoxetine, paroxetine, and citalopram in different cohorts of unipolar, bipolar, and psychotic depression patients (127–132). Japanese and Korean patients in the two exceptions of the nine had an opposite direction of response association and also had LL genotype frequencies of only 7% compared to ~30% in most studies in Caucasians; this may have contributed to the clinical response discrepancy (127, 129, 130). In addition, one serotonin receptor, 5HT2, possesses two variants that occur in different frequencies in Japanese vs European groups (131); also, CYP2D6-dependent drug metabolism differs between Asians and Caucasians (132).
Curiously, two studies of responses to SRIs in patients with OCD did not observe associations between the SERT 5 HTTLPR genotypes and therapeutic responses (133, 134). Whether or how this might relate to reports of more frequent LL genotypes in OCD or to the fact that patients with OCD only respond to SRI antidepressants, whereas depressed patients respond to all types of antidepressants, including norepinephrine reuptake inhibitors and MAO inhibitors, remains to be evaluated (122, 135). Of interest, mice with genetically reduced or absent SERT failed to demonstrate the usual “antidepressant responses” in helplessness-related behaviors (e.g., immobility in a swim test), although responses to other non-SRI antidepressants were unchanged (136). One explanation about why humans with the SS genotype and mice with SERT mutations have deficient responses to antidepressants is that a true “floor” effect may be responsible––that is, further SRI-dependent inhibition of SERT activity might not be possible.
Regarding adverse reactions observed during SRI treatment of depression, a 3.5-fold greater incidence of insomnia (78% vs 22%) and a 9.5-fold greater incidence of agitation (67% vs. 7%) but not other side effects including nausea or headache were found during treatment with fluoxetine in individuals with the SS 5HTTLPR genotype compared to SL or LL individuals (137). Similarly, a 5.3-fold higher incidence of hypomania as a side effect occurred in bipolar patients receiving “serotoninergic” (mostly SRI) antide-pressants who possessed the SS genotype vs the other genotypes (138). A subsequent study found an association between the SS genotype and a rapid cycling bipolar course of illness (four or more episodes per year) in individuals in an SRI study, but not a direct association between the SS genotype and hypomania, raising the question as to whether the original finding was reflective of a clinical subgroup rather than a pharmacogenetic phenotype (139). Suicide attempts, which represent a controversial possible side effect of SRIs, were found to be associated with the SS genotype and S allele in a recent large review (78).
If further studies continue to support these treatment and side effect associations, 5HTTLPR genotyping may prove to be clinically important in avoiding lengthy treatment trials with one and then a second SRI (as some current guidelines for antidepressant treatment with SRIs suggest) before considering another drug class or treatment modality. One conjecture about mechanism underlying both the possible enhanced susceptibility to hypomania and to impulsive, violent suicide is that serotoninergic modulation is diminished in SS individuals, leading to unrestrained activation, perhaps via catecholamine influences, as suggested some years ago for “switches” into mania (140–142).
In addition to the SERT polymorphism-SRI interactions, a nucleotide polymorphism in the 5HT2A receptor (T102C variant), one of the 14-plus post-synaptic serotonin receptors at which increased serotonin concentrations produced by SRI administration acts, has also been found to be strongly associated with intolerance to paroxetine. Subjects with this variant discontinued paroxetine early during the double-blind study and exhibited significantly greater side effect severity (143). SERT genotyping was not reported in this study, and other possible interactions between SERT variants and above-mentioned serotonin receptor gene variants have not yet been published.
Additional considerations that do not seem to have been addressed thus far are that one or more genes that interact with SERT alleles to yield altered neurochemistry and behavior in mice might be involved epistatically in humans (82). These include genes that encode DAT, NET, MAOA, and BDNF, all of which have functional variants in humans (82). For example, it is plausible that uncommon side effects such as the serotonin syndrome––which includes dilated pupils, facial flushing, diaphoresis, shivering, myoclonic jerks, and tremors––or reactions to tyramine-containing products, or even other toxically mediated behaviors observed in SRI or MAOI-treated individuals might represent specific consequences of SERT × MAOA interactions (144, 145). In the future, more extensive genotyping using chip may become possible. More extensive ascertainment of life stresses and other environmental factors that may contribute to increased vulnerability to adverse events in susceptible individuals or subgroups may assist difficult decision-making in clinical practice, such as that found in the currently much-debated treatment of pediatric patients with SRIs (79, 146). It is possible that correct evaluation of the association between suicide attempts and SRI-treated sub-groups may depend upon knowing the frequency of especially vulnerable individuals, such as those with the SS 5HTTLPR genotype, in each study.
Conclusion
Recent results from pharmacogenetic studies using the SRIs fluoxetine, paroxetine, citalopram and fluovoxamine in >850 individuals in nine studies genotyped for the SERT 5HTTLPR polymorphism suggest that the time may be nearing to consider clinical use of such genotyping. Uniformity in the design of recent studies and the incorporation of proper controls (such as the use of placebo groups and alternative antidepressant drugs) has been lacking. Thus, a large prospective study may be desirable to ascertain whether the available results indicating that the SS 5HTTLPR genotype is associated with significantly poorer antidepressant responses and more frequent major side effects are correct. Once DNA is obtained from patients planned for SRI treatment, concomitant genotyping for drug metabolizing enzyme polymorphisms (when this becomes feasible) may also help minimize the number of lengthy (weeks to months) trials with these drugs that have slow time courses for therapeutic efficacy. Similarly, such 5HTTLPR genotyping could be of major assistance in investigations using new PET ligands for SERT, such as [11C]-DASB) in second generation studies of human brain SERT in serotonin-related diseases and of their treatment with SRIs and serotoninergic receptor agents. In fact, it is now recognized that a long list of diseases and neuropsychiatric disorders are highly likely to have genetic contributions from SERT. These include developmental disorders like ADHD, autism, sudden infant death syndrome (SIDS) and Tourette’s syndrome as well as equally complex disorders such as polysubstance abuse, IBS, PPH, and myocardial infarction. Vulnerability for these disorders often appears to be conferred by the greater-expressing L allele, especially the homozygous LL genotype, and vulnerability may even be enhanced by the presence of super-long 5HTTLPR alleles in the case of SIDS (147) and the case of a constitutive gain-of-function mutation in rare familial OCD (55, 56). All of these disorders are recognized as genetically complex, with multiple gene × gene (as well as environmental) interactions. Thus, the highly evolutionarily conserved serotonin transporter, which regulates the entire serotoninergic system including the fourteen or more receptors associated with it via changes in extracellular fluid serotonin concentrations, has come to center stage as an essential modulator in brain, gut, heart, lung, platelets, endocrine glands and other tissues, with newly defined roles in many genetically complex disorders and in their treatment.
Acknowledgments
We thank Robert Innis, MD, PhD, and Sevilla Detera-Wadleigh, PhD (NIMH), Andrew Holmes, PhD (NIAAA), Michael D. Gershon, MD (Columbia University), Anne A. Andrews, PhD (Pennsylvania State University) and Stephanie Watts, PhD (Michigan State University) for helpful comments about earlier drafts of the manuscript. We are indepted to Ms. Theresa B. DeGuzman, Ms. Su-Jan Huang and Ms. Teresa Tolliver (NIMH) and Ms. Kiara Cromer (University of Florida, Tallahassee) for multiple editorial, artwork, and scientific contributions.
- © American Society for Pharmacology and Experimental Theraputics 2004
References

Klaus-Peter Lesch, MD, is a Professor and Vice-Chair in the Molecular and Clinical Psychobiology and Director of the ADHD Program in the Department of Psychiatry and Psychotherapy of University of Wuerzburg, Germany.

Gary Rudnick, PhD, is a Professor in the Department of Pharmacology of Yale University School of Medicine, New Haven, CT.

Alicja Lerner, MD, PhD, is a Senior Research Fellow in the Laboratory of Clinical Science.

Dennis L. Murphy, MD, is Chief of the Laboratory of Clinical Science, National Institute of Mental Health, NIH, Bethesda, MD.