Olfactory Receptor Localization and Function: An Emerging Role for GPCR Heterodimerization
The mammalian sense of smell represents one of the most complex and intricate physiological systems in the body and is essential for taste perception, sexual attraction, and avoidance of danger. The detection of specific odorants begins with the stimulation of olfactory receptors (ORs)––humans possess more than 350 ORs, whereas mice have more than 1000 ORs––on sensory neurons within the olfactory epithelium of nasal cavities, resulting in the propagation of signals to the main olfactory bulb. ORs are members of the G protein coupled receptor (GPCR) superfamily, cell surface proteins with a conserved structure of seven transmembrane domains. The importance of this large GPCR family was recently emphasized by the bestowal of the 2004 Nobel Prize in Physiology or Medicine to Richard Axel and Linda Buck for the discovery of olfactory receptors.
Although ORs were initially cloned over twelve years ago (1), little is presently known about the properties or ligand specificities of most of them. This is primarily because ORs, like a number of other GPCRs, do not efficiently reach the cell surface following heterologous expression in most cell lines in vitro. Modifications of ORs have been used to facilitate their cell-surface expression, including addition of N-terminal epitope tags (2), rhodopsin N-terminal domains (3), or the creation of chimeras between ORs and β2 –adrenergic receptors (β2 ARs) (4). Recently, however, a report by Larsson et al. (5) implicated receptor heterodimerization, an emerging concept in GPCR pharmacology, as a determining factor for proper expression, localization, and function of ORs.
Although the concept of GPCR heterodimerization had been hypothesized for some time, the functional importance of this phenomenon remained a mystery for many years. However, with the discovery that functional γ-amino butyric acid subtype B (GABAB) receptors (GABABR) require the obligatory assembly of two distinct members of the small Class III GPCR subfamily, specifically, GABABR1 and GABABR2, to form a single functional receptor (6), it became clear that heterodimerization is critical for proper trafficking and expression of certain GPCRs. Additionally, the hypothesis is supported by the finding that the T1R1 family of taste receptors (i.e., T1R1, T1R2, and T1R3)––also members of the Class III GPCR family––require the obligatory assembly of two distinct T1R GPCRs to form a single functional receptor (7). Similar complexes occur among other members of the Class III family (8) and also happen between members of the much larger Class I (rhodopsin) GPCR subfamily (9). Thus, it is clear that GPCRs can exist as dimers or multimeric complexes, and, in some cases, heteromeric interactions between distinct receptors are required for proper expression, pharmacological specificity, and function.
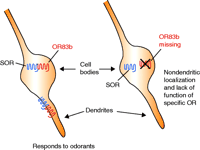
In their recent study, Larsson et al. implicated the importance of a single OR in imparting the ability to detect and respond to odors in Drosophila melanogaster (5). The odor-response profile of Drosophila is governed by the selective expression of various members of a family of sixty-one OR genes. Unlike mammalian olfactory neurons, each of which probably express only a single OR (10), essentially all Drosophila olfactory sensory neurons (OSNs) express no less then two distinct ORs, all from the Class I subfamily. One OR, termed OR83b, is detected in all 120 adult maxillary pulp neurons and in 70–80% of all antennal OSNs. OR83b is an atypical GPCR that has little sequence homology with and is significantly larger than the other sixty Drosophila ORs. Because OR83b is always coexpressed with another OR in OSNs, previous studies found it difficult to characterize the odor-response profile of OR83b. Thus, Larsson et al. created Drosophila strains lacking OR83b to examine this question. Amazingly, OR83b null mutants suffered severe disruptions in their entire olfactory system. In adult OSNs, conventional ORs were not properly localized to their specific dendritic compartments (Figure 1⇓). Electrophysiological functional responses stimulated by eight different odors were completely abolished in the adult antenna of OR83b knockout strains. In addition, OR83b-deficient flies did not demonstrate odor-evoked behavior in the larval or adult stages. From these experiments, the authors concluded that OR83b is essential for odorant detection in Drosophila by promoting the localization and functional activity of other ORs. Thus, although biochemical evidence for receptor heterodimerization has not yet been demonstrated in this system, these experiments strongly suggest that OR83b can act as a chaperone receptor in Drosophila, probably through heterodimerization with other ORs to ensure their appropriate dendritic localization and function (5).
A second, independent line of evidence for OR heterodimerization has come from our recent experiments on the trafficking and functional activity of a mouse OR termed M71 (11). Using various epitope tags and detection methods, we found that mouse M71 ORs are retained intracellularly (like other mammalian ORs) when expressed heterologously in HEK-293 cells; however, M71 ORs coexpressed with β2 ARs are quantitatively translocated to the cell surface in a subtype-specific manner. Coimmunoprecipitation and cointernalization experiments revealed that M71 OR and β2 AR associate in response to activation with specific agonists. Also, coexpression of wild type M71 ORs with β2 ARs in cells treated with an M71 OR agonist resulted in increased adenosine 3′, 5′ monophosphate (cyclic AMP) production, suggesting that the receptors become functional upon heterodimerization. Thus, like the findings of Larsson et al. (5), these data support the idea that GPCR heterodimerization may generally be an important mechanism in controlling OR localization and function.
These two recent and complementary studies, encompassing work in both Drosophila and in mammals, suggest that ORs can be added to the growing list of intracellular GPCRs that require specific GPCR partners for chaperoning to the cell surface. A recent report by Salahpour et al. (12) suggests that β2 ARs must first form multimeric complexes in the endoplasmic reticulum in order to be expressed on the cell surface. This raises the possibility that many, if not all, GPCRs must form such multiprotein complexes to facilitate their surface expression. For some receptors, such as β2 ARs, homodimeric associations may be sufficient to allow for trafficking to the plasma membrane, whereas for other receptors, such as ORs, interactions with other specific receptor types may be required for surface expression.
The inability of ORs to traffic to the cell surface following heterologous expression has been an enduring problem for more than a decade, resulting in difficulties in defining the ligand specificities and functional activity of this huge receptor family. At present, specific ligands are known for only a very small number of ORs. Thus, coexpression of ORs with other GPCRs may serve as a general mechanism for obtaining OR surface expression and responsiveness in heterologous cells, allowing for more detailed analysis of this enormous and extremely important GPCR family.
Schematic illustrating the role of OR83b in specific olfactory receptor (SOR) localization and function in Drosophila olfactory sensory neurons. In the presence (left) of the widely expressed olfactory receptor OR83b, both the conventional SOR and OR83b are properly localized to their specific dendritic compartments at the cell surface, whereas, in the absence of OR83b (right), the SOR remains localized intracellularly.
- © American Society for Pharmacology and Experimental Theraputics 2004
References

Chris Hague, PhD, (right) is currently a postdoctoral fellow in the laboratory of Dr. Minneman. E-mail chague{at}emory.edu
Randy A. Hall, PhD, (center) is an Assistant Professor in the Department of Pharmacology at the Emory University School of Medicine. E-mail rhall{at}pharm.emory.edu
Kenneth P. Minneman, PhD, (left) is Charles Howard Candler Professor of Pharmacology at the Emory University School of Medicine. E-mail kminneman{at}pharm.emory.edu; fax 404-727-0365.