The Ileocyte Basolateral Organic Solute Transporter (OSTα–OSTβ) Complex: Finding The Missing Link in Enterohepatic Circulation
Biliary excretion is an important route of elimination for many drugs, their conjugates, or both, and some compounds excreted into bile may be reabsorbed in the intestine. Bile acids are excreted and reabsorbed with ≥ 95% efficiency through a process called enterohepatic circulation (1, 2). The occurrence of enterohepatic circulation has long been known, but its mechanisms have only recently been elucidated.
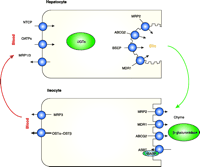
In enterohepatic circulation, bile acids must cross four membranes: the basolateral and apical (canalicular) membranes of both the ileocyte and the hepatocyte. More than 80% of conjugated bile acids are taken up into the liver from portal blood predominantly by the Na+-taurocholate cotransporting polypeptide [NTCP, also termed solute carrier (SLC) 10A1] (3), and uptake of the remainder is mediated by the organic anion transporting polypeptide [OATP, also termed solute carrier Oatp subfamily (SLCO)] family members (2). Alternatively, bile acids can be synthesized de novo exclusively in the hepatocyte. Bile acids are then transported across the canalicular membrane during bile formation by the bile salt export pump [BSEP, also termed ATP-binding cassette (ABC) subfamily B protein 11 (ABCB11)]; bile is secreted into intestinal chyme, and bile acids are reabsorbed into the ileocyte by the apical sodium-dependent bile salt transporter (ASBT, also termed SLC10A2) (2). The mechanism by which efflux across the basolateral membrane from the ileocyte into the portal blood, however, has remained unclear. The likelihood that multidrug resistance–associated protein 3 [MRP3, also termed ABC subfamily C protein 3 (ABCC3)] is the principal basolateral bile acid transporter––responsible for basolateral efflux of bile acids from the ileocyte––was refuted by recent evidence indicating that MRP3 has low affinity for bile acids (4); furthermore, Mrp3 (Box 1) mRNA expression is relatively low in mouse ileum (5).
Transporter Nomenclature: Roses by Several Names
Acronyms that are all capitalized indicate the human form of the protein, whereas acronyms with initial capitals followed by lowercase letters are used for mammalian and other species including the little skate. Both the common and the standard (HUGO) names and acronyms are provided in the text. With the exception of ABCG2 (a standard acronym), common acronyms are used in Figure 1⇓. To limit confusion, we have avoided ABCG2’s three common acronyms: ABCP, MXR, BCRP––coined by three groups independently working on the same protein. OSTα–OSTβ will also be assigned a standard name. Readers may refer to The Human Genome Organization’s (HUGO) Gene Nomenclature Committee (HGNC) Web site (http://www.gene.ucl.ac.uk/nomenclature/genefamily.html) which contains a useful search feature for protein family nomenclature.
The transcriptional profiling approach used by Dawson et al. has proved effective in identifying the candidate protein(s) responsible for basolateral bile acid transport in the ileocyte (5). They hypothesized that the expression of the transporter would be high in the ileum and would be stimulated by bile acids. Dawson et al. used Slc10a2−/ − mice, deficient in Asbt, anticipating that decreased concentrations of bile acids in ileocytes and increased (10-fold) bile acid flux through the cecum and colon would decrease (in ileocytes) and increase (in cecum and colon) the expression of the basolateral transporter (5). They found that expression of the mouse ortholog of the organic solute transporter alpha (Ostα) subunit was greatly increased in the colon of Slc10a2−/ − mice. Interestingly, Ostα and Ostβ were discovered in a primitive marine vertebrate, the little skate (Leucoraja erinacea), through an expression cloning search for novel Oatps (6).
Dawson et al. showed that in mice, the expression of Ostα and Ostβ closely parallel each other and that of Asbt, but not that of Mrp3 (5). Ostα is composed of 340 amino acids, and is predicted to have seven transmembrane–spanning domains, whereas Ostβ has 128 amino acids, and a single transmembrane domain. Their amino acid sequences are not similar to other previously identified solute carrier (SLC) gene family members, indicating that Ostα and Ostβ form a unique transporter. The authors transiently transfected human embryonic kidney (HEK) 293 cells and stably transfected Madin-Darby canine kidney (MDCK) cells, finding that coexpression of Ostα and Ostβ is required for mature glycosylation, membrane targeting, and taurocholate transport activity of the complex. The requirement for two distinct proteins from distinct genes is uncommon, but has been compared to the architecture of the heterodimeric Na+-K+–ATPase, in which the α-subunit contains ten membrane-spanning domains and provides the transport and catalytic activities, whereas the β-subunit has only one membrane-spanning domain but is required for pump assembly and targeting to the membrane (6). Furthermore, despite only 25–41% amino acid identity in OSTα and OSTβ shared between humans and little skate, all nine combinations of the α and β peptides from human, mouse, and little skate transported estrone-3-sulfate (7).
Dawson et al. have identified the Ostα–Ostβ complex as a major contributor to bile acid recirculation by identifying its basolateral location and its transport of taurocholate. The Ostα–Ostβ transporter is saturable and sodium-independent; it transports a range of substrates similar to those carried by the Oatp transporters found on the basolateral membrane of the hepatocyte, including taurocholate, estrone-3-sulfate, dihydroepiandrosterone sulfate, digoxin, and prostaglandin E2 (7). The contribution of OSTα–OSTβ to bile acid recirculation in humans, the range of its substrate specificity and selectivity, especially regarding drugs, as well as the regulation of the complex’s expression are important topics for future investigations.
Some drugs, such as imipramine, indomethacin, morphine, and pregnenolone may also undergo enterohepatic circulation, in which the parent drugs or their glucuronide conjugates may follow a pathway similar to the one used by bile acids (1). Following systemic blood circulation, drugs undergoing enterohepatic circulation would be taken up into the hepatocyte (by NTCP or OATPs), glucuronidated [by uridine-5′-diphosphate (UDP)-glucuronosyltransferases], then excreted into bile by MRP2 (ABCC2), ABCG2, and/or MDR1 (ABCB1) (Figure 1⇓). β-Glucuronidase, present in bacteria inhabiting the gut, hydrolyzes the glucuronide conjugate back to the parent compound, which is then reabsorbed. Although MDR1, MRP2, and ABCG2 are positioned apically on the ileocyte and oriented to prevent further drug absorption (via the basolateral side into the portal blood), upon the entrance of a drug into the ileocyte, basolateral membrane–localized transporters such as OSTα–OSTβ and MRP3 could facilitate drug transport into portal blood (2). The impact of Ostα–Ostβ and MRP3 on enterohepatic circulation of drugs remains to be established; however, OSTα–OSTβ is probably much more important than MRP3 for recirculation of bile acids. Further studies will elucidate the role of OSTα–OSTβ in enterohepatic circulation and drug absorption, allowing for new strategies in exploiting these pathways for transporter-mediated drug delivery.
Simplified schematic depiction of transporters involved in enterohepatic circulation of bile acids and drugs. Arrows (black) indicate the direction of substrate movement through transmembrane transporters. Drugs undergoing enterohepatic circulation are taken up into the hepatocyte by NTCP or OATPs. Once inside the cell, drugs may be glucuronidated by UGTs, then excreted into bile by MRP2, ABCG2, and/or MDR1. β-Glucuronidase, present in bacterial flora in the gut, hydrolyzes the glucuronide conjugate back to the parent compound, which is then reabsorbed by ileocytes (2, 3). OSTα–OSTβ is thought to have a much more important role than MRP3 in impacting basolateral bile-acid transport in ileocytes (5). The ileal bile acid binding protein (IBABP) is cytoplasmically attached to ASBT in a multimeric complex and plays an important role in intracellular binding and trafficking of bile acids (2, 3). NTCP, Na+-taurocholate cotransporting polypeptide; OATP, organic anion transporting polypeptide; MRP, multidrug resistance–associated protein; ABCG2, ATP-binding cassette, subfamily G protein 2; BSEP, bile salt export pump; MDR1, multidrug-resistance 1; OSTα–OSTβ, organic solute transporter alpha and beta complex; UGTs, uridine-5′-diphosphate (UDP)-glucuronosyltransferases; IBABP, ileal bile acid binding protein.
Acknowledgments
The authors acknowledge the assistance of Ms. Laura Lengowski in the preparation of the figure.
- © American Society for Pharmacology and Experimental Theraputics 2005
References

Mary Vore, PhD, is Professor and Director, Graduate Center for Toxicology at the University of Kentucky College of Medicine. She received her PhD in Pharmacology from Vanderbilt University, then trained in the biochemistry of cytochrome P450s with Dr. Anthony Lu at Hoffman-LaRoche, Inc. Dr. Vore’s laboratory focuses on the regulation of expression and activity of hepatic bile acid and organic anion transporters in pregnancy and during lactation.

Phillip M. Gerk, PharmD, PhD, is an Assistant Professor of Pharmaceutics, Virginia Commonwealth University School of Pharmacy (Medical College of Virginia campus). He received his PharmD from the University of Illinois at Chicago in 1993, completed a postdoctoral fellowship at Auburn University School of Pharmacy from 1993 to 1995, then received his PhD in Clinical Pharmaceutical Science at the University of Kentucky in 2000, working with Patrick McNamara. From 2000–2004, he completed a postdoctoral fellowship in Toxicology with Mary Vore. In 2004 he joined the faculty at VCU.