Nascent Transcripts
New Platinum Complexes for the Anti-Cancer Armamentarium
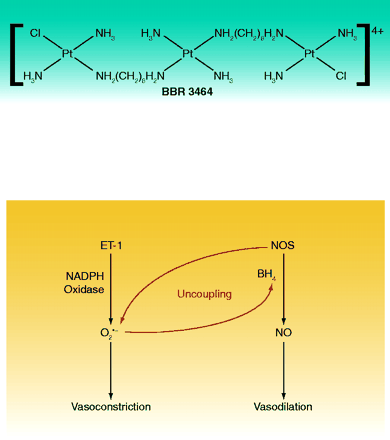
On December 19, 1978, the FDA approved cis-diamminedichloroplatinum (better known as cisplatin) for human cancer treatment. Subsequent work has suggested that the cis configuration is essential to satisfy structural requirements for DNA adduct formation. Harris et al. now challenge this dogma by synthesizing compounds that are distinct from clinically used agents such as cisplatin and oxaliplatin. The authors describe how a novel trinuclear platinum-based compound, BBR 3464, interacts with DNA in a manner that also includes a noncovalent binding component—a process quite distinct from that utilized by cisplatin or oxaliplatin. BBR 3464 and other more highly charged congeners that only undergo noncovalent interactions induce caspase-dependent apoptosis in both primary mast cells and in transformed mastocytomas. Surprisingly, cellular uptake of the more highly charged compounds is enhanced. These findings may form the basis of a future class of anti-cancer agents. [
] —JS Lazo, U Pittsburgh
Enhanced Endothelin-Mediated Vasoconstriction by Uncoupling NOS
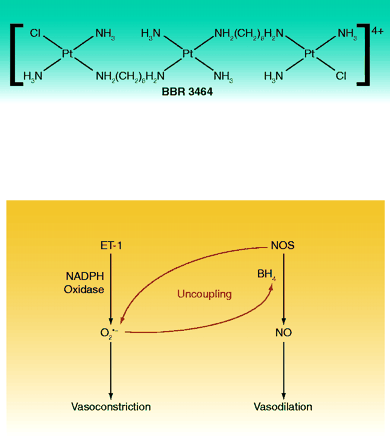
Endothelin (ET-1) stimulates vascular ETA and ETB receptors, leading to increased superoxide (O2•−) production and vasoconstriction. This is normally counterbalanced by activation of endothelial nitric oxide synthase (NOS), which uses its cofactor tetrahydrobiopterin (BH4) to produce NO and thus result in vasodilation. On the other hand, oxidation of BH4 by NADPH oxidase causes the uncoupling of NOS and the production of O2•−, but the role of this divergent pathway in ET-1-induced vasoconstriction is unclear. Pollock and colleagues investigate a possible link between ET-1-mediated vasoconstriction and the O2•− produced by NOS uncoupling. Using rat aortic rings, the authors find that ETA/ETB antagonists, NADPH oxidase inhibitors, and BH4 inhibit both ET-1-induced O2•− production and enhancement of contraction. These findings suggest that ET-1, through ETA/ETB receptors, stimulates NADPH oxidase, BH4 oxidation, and NOS uncoupling, leading to enhanced O2•− production and vasoconstriction. By minimizing the uncoupling of NOS, researchers may develop a new approach to reduce ET-1-mediated dysfunction of NOS regulatory pathways and consequent vascular disorders. [
] —RA Khalil, Harvard Medical School
Nerve Growth Factor: An Inducer of Endothelial Cell Migration
The formation of new blood vessels (i.e., angiogenesis) is essential to embryonic development and normal physiology and to the development and maintenance of pathophysiological states, including diabetes and cancer. In response to an angiogenic growth factor, endothelial cells migrate into the interstitial space and generate new vessels. Endothelial cell migration is a pharmacological target for drug discovery: Vascular endothelial growth factor (VEGF) is known to induce migration of endothelial cells, an effect mediated by activation of receptor tyrosine kinases. Dollé et al. report that nerve growth factor (NGF), a neurotrophin that participates in the function of sympathetic and sensory nervous systems, induces the migration of endothelial cells in vitro. This implies that NGF may represent a novel angiogenic factor with an important role in the cardiovascular system and may thus be relevant for the wide-spread crosstalk between the nervous and cardiovascular systems in vivo. [
] –R Levi, Weill Cornell Medical College, New York, NY
Agonizing on the hERG Cardiac K+ Channel
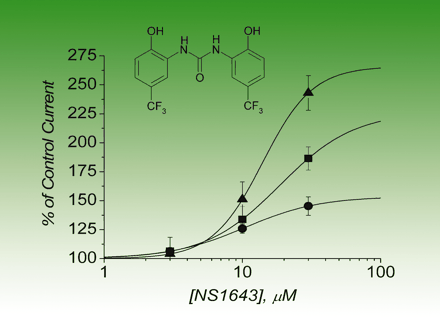
The hERG (human ether-a-go-go-related gene) cardiac K+ channel participates in controlling repolarization of the human heart, and inhibition of its function—either through drug blockade or congenital defects—can lead to cardiac arrhythmia and sudden death. Although many drugs can antagonize the channel, only recently have reports begun to describe compounds that work as activators of hERG. Casis et al. explore the pharmacological activity of one of these agents, NS1643, and reveal that the main mechanism of action leading to increased channel function is a reduction in hERG channel inactivation. By using mutant hERG channels, the authors identify a weak antagonistic activity of the drug, leading the conclustion that NS1643 is best classified as a partial agonist. These studies represent some of the first steps towards characterizing a new pharmacological class of drugs, the hERG channel activators. These compounds will likely be valuable tools for exploring the structure and physiological function of the hERG channel and may one day find use in treating arrhythmia or other diseases. [
] —D Rampe, Sanofi-Aventis, Bridgewater, NJ
- © American Society for Pharmacology and Experimental Theraputics 2005



