When She Says Yes, But He Needs NO: Some Reflections on Nitric Oxide
Shortly after the 1998 Nobel Prize in Medicine or Physiology was announced, a niece of mine indicated that she was angered, though not surprised, that the prize had been given to reward the creators of Viagra®. Indeed, this was the spin put on the news by the late night TV comedians (1). I explained that Robert Furchgott, Louis Ignarro, and Ferid Murad had nothing to do with developing the drug; that they had elucidated the physiologic effects of nitric oxide as a signaling agent in the cardiovascular (CV) system; and that their discovery had broad potential implications for the treatment of many disorders. My niece, however, felt that it was not purely by chance that the first practical application was a drug that would boost the egos of the male sex.
Setting aside gender-related issues, there are many interesting facets to the nitric oxide (NO) story. This article takes a circuitous route: it briefly recounts the discovery of NO’s amazing physiologic functions, then discusses a number of NO-releasing medicinals that were used for a century or more before their mode of action was known, and returns finally to the present-day medical applications of the nitric oxide discovery.
Molecule Of The Year
What is nitric oxide, and why did the editors of Science honor it as the Molecule of the Year 1992 (2)? In the 18th century, Joseph Priestley characterized nitric oxide as one of several oxides of nitrogen. Its unsaturated electronic structure makes it highly reactive. Indeed, Priestley used it in his experiments to remove residual oxygen from other gases and to measure the oxygen content of air. NO reacts rapidly with oxygen, forming the brown gas NO2, which is soluble in water (3).
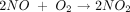
In the twentieth century NO was mainly associated with air pollution, as it is found in auto exhaust, tobacco smoke, and smog. NO also catalyzes the conversion of ozone (O3) to molecular oxygen (O2) in the atmosphere, thus contributing to the depletion of the ozone layer.
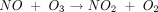
Who would have thought that this “nuisance” is produced by human cells and exerts a variety of effects, for good and for ill!
The timeline of the Nobel-winning discovery has been outlined as follows (4). In 1977, Ferid Murad discovered that nitroglycerin acts on vascular smooth muscle by releasing NO. In 1980, Robert Furchgott and John Zawodski recognized that endothelial cells release a substance which promotes acetylcholine (AC)-induced vasorelaxation. Its chemical nature being unknown, it was named endothelium-derived relaxing factor (EDRF). In 1987, Louis Ignarro and Salvador Moncada identified EDRF as identical to NO.
Murad’s group spent twenty-five years studying the synthesis and function of cyclic guanosine 3′,5′ monophosphate (cGMP). Finding that diverse organic and inorganic nitrites and nitrates caused cGMP concentrations to increase, followed by smooth muscle relaxation, the research group suggested that NO could be the common active agent produced by these compounds. Murad coined the term “nitrovasodilators” for compounds that can be converted to NO enzymatically or nonenzymatically (5).
Furchgott’s contribution came from his use of isolated strips of guinea pig tracheal smooth muscle and rabbit aorta for quantitative pharmacologic studies on vasoactive drugs, including the relaxant effect of AC. He noted that unless these strips were prepared with the utmost care to avoid rubbing the intimal surface, the AC effect was abolished; rubbing removed endothelial cells. Furchgott’s group then found that endothelial cells in vitro, when stimulated by AC, release a substance that acts on the smooth muscle of blood vessels causing relaxation. This substance––diffusible, very labile, and of unknown structure––they named EDRF. Several years later, convincing evidence was developed that EDRF and NO were identical (6).
The chemical messengers hitherto recognized had all been organic amine compounds (e.g., acetylcholine, epinephrine), thus, it was hard for the research community to accept that NO, a simple inorganic compound, possessed similar activity. Added to that difficulty was the minute concentration of NO involved: the apparent activation constant required for activity was estimated by Murad to be in the 1–10 nmol/L range (5). However, by the 1980s, physicochemical methodology was available to demonstrate the formation of NO and quantitate it. Palmer described a chemiluminescence procedure which correlated with bioassay results on rabbit aorta (7). Endothelial cells in a column were treated with bradykinin to release NO. Effluent from the column was passed into an intermediate reaction vessel, and NO was then removed in a nitrogen stream under reduced pressure. Reaction of NO with ozone yielded a chemiluminescent product, which was measurable with a photomultiplier. Liberated NO was measured in the range of 0.07 to 0.67 nmol. A mass spectrometry method for the measurement of NO has also been published (8).
Nitrovasodilators
Nitrites
We have long used the effects of NO in therapeutics without knowing it. Before the advent of chlorothiazide (Diuril®) and other effective antihypertensives, very little was available to lower blood pressure. The sedative phenobarbital was often prescribed in the hope that calming the patient might reduce his high blood pressure. Sodium nitrite, which acts to relax the smooth muscles, especially of small blood vessels, was used in the treatment of hypertension. It can also be used intravenously as an antidote for cyanide poisoning. Nitrites convert hemoglobin to methemoglobin, which combines with cyanide and inactivates it temporarily as cyanmethemoglobin. Sodium thiosulfate is then injected, forming relatively nontoxic thiocyanate (9).
Ironically, the same hemoglobin-nitrite reaction can be a source of nitrite toxicity. Berton Roueche’s medical mystery Eleven Blue Men relates how gravely ill men were brought to a New York hospital, blue from cyanosis. Public health officials determined that they had been poisoned after eating oatmeal because salt shakers in their Bowery cafeteria had accidentally been filled with sodium nitrite (10).
In the early days of synthetic organic chemistry, various alcohols were reacted with sodium nitrite and sulfuric acid to yield nitrite esters. Ethyl and amyl nitrites, in particular, have a history of medicinal use. Ethyl nitrite, a volatile liquid boiling at 17°C, was used as a 4% solution in alcohol called ethyl nitrite spirit. Among lay people it was known as sweet spirits of nitre or simply sweet nitre. In my own childhood recollection, its taste was not sweet, but horrific. Its use dates back at least to mid-19th century, and in the 1930s and 1940s a one-ounce bottle of sweet nitre was still present in the typical home medicine cabinet. Although ethyl nitrite shares the general actions of nitrites (causing lowering of blood pressure, facial flushing, giddiness, nausea, headache), sweet nitre was used mainly as a diaphoretic, to induce sweating and thereby reduce fever in children. It was given to infants to relieve intestinal colic. Depending on the child’s age, the dosage ranged from several drops to two milliliters. It was also applied topically for cold sores and fever blisters (11).
Although its popularity had waned, it was still being used in the 1970s. Then trouble arose. Two deaths, one of an infant and one of a three-year-old child, were reported to the Food and Drug Administration (FDA). Sixteen nonfatal poisonings of children had been reported between 1969 and 1973. Absent definitive proof of effectiveness, these toxicity concerns led an OTC Advisory Review Panel to conclude that sweet nitre is neither safe nor effective. The FDA then banned the product as of June 27, 1980 (12).
Amyl nitrite (actually, isoamyl nitrite) is a vasodilator with a very rapid onset and short duration of action. In 1865–1867, Sir Thomas Lauder Brunton brought amyl nitrite into wide-spread use, by inhalation, for relief of angina (13). A glass “pearl” containing 0.2 mL, held inside a handkerchief, was crushed, and the volatile liquid inhaled. It was also occasionally used in emergency treatment of cyanide poisoning, in which condition it acts like sodium nitrite.
Neither a nitrite nor a nitrate, but an inorganic complex incorporating NO in its molecule, is sodium nitroprusside, Na2[Fe(CN)5NO]. Known since 1850, its hypotensive effect in humans was described in 1929, but its therapeutic use was not elucidated until the 1950s. It is used by intravenous (iv) injection to control hypertensive crises and to improve cardiac function in left ventricular failure (14). It is available in sterile solutions containing 25 mg/mL. Following iv infusion its effects start within seconds, lasting only 1–10 minutes. Sodium nitroprusside treatment should not be continued for more than seventy-two hours, as this compound releases cyanide, which can be metabolized with limited success by the body to thiocyanate.
Nitrates
The most significant of the nitrates is probably glyceryl trinitrate, or nitroglycerin (NG). Discovered in 1847 by an Italian chemist, its ability to cause violent headaches was quickly noted. It was suggested as a possible homeopathic headache remedy, under the doctrine of “like cures like.” In 1851, Alfred Nobel began working with NG and he was able to solve the handling problems, turning its explosive properties to practical use. NG became the source of his vast fortune, which now funds the Nobel Prize (4).
As related by Sneader (15), the medicinal use of NG was made possible by the willingness of a medical man to act as a “lab-rat”. William Murrell, of London’s Westminster Hospital, started out to resolve the conflicting literature reports as to whether NG caused severe headache. He tested NG on himself at least thirty times before persuading friends and other volunteers to participate in a trial of its effects. Using the sphygmograph––at the time a new instrument––to follow changes in blood pressure, he discovered that NG’s onset of action was 2–3 minutes and its duration of effect about thirty minutes. This contrasted to amyl nitrite, which acts in ten seconds but wears off in five minutes. Murrell concluded that NG might be superior for treating angina. He found that the
NG headache arose from overdosage, that the effective dose was 0.5–1 mg, and that NG tablets should be allowed to dissolve in the mouth, not swallowed. Murrell treated his first patient in 1878 and in 1879 published his first report, which resulted in the adoption of NG into clinical practice in England.
NG posed a number of pharmaceutical formulation problems owing to its volatility, low dosage (0.65 mg), and the need to administer it under the tongue for rapid relief. Perhaps the first special formulation was William Martindale’s tablet using chocolate as the carrier. Although good tasting, it proved unstable and the standard dosage form became a tiny sublingual tablet with a lactose or mannitol base. More recently, NG became one of the first drugs to be administered transdermally; available as a 2% ointment in 1988 and later in the far more convenient patch. It is also marketed in a metered-dose spray container, with each spray delivering 0.4 mg of NG on or under the tongue (16).
Newer Nitrates
Seeking antianginals with a longer duration of action, medicinal chemists prepared fully nitrated esters of higher polyhydric alcohols (i.e., polyols). Erythritol, with four carbons and four hydroxyls, yielded erythrityl tetranitrate, with a peak effect at thirty minutes and duration of 2–4 hours.
The six-carbon analog, mannitol hexanitrate, has an onset of 15–30 minutes and duration of 4–6 hours. Pentaerythritol tetranitrate (PETN) has an unusual structure: instead of the chain structure found in NG and the above two analogs, all four –CH2OH groups of pentaerythritol are attached to a single carbon atom. PETN’s effects have an onset of approximately one hour and duration of about five hours (17, 18). Both of the erythritol esters were used for prophylaxis against angina pectoris attacks rather than to abort the attacks. Mannitol hexanitrate is not very effective against angina and was used for hypertension instead (17, 18). Like NG, the other nitrates are explosive; PETN, in fact, is used industrially in the manufacture of detonating fuses. For processing into tablets, the nitrates are sold well-diluted with lactose.
Other than NG itself, the polynitrates have been superceded by isosorbide mononitrate (ISMN) and dinitrate (ISDN). Isosorbide is a bicyclic anhydride of the six-carbon polyol sorbitol and has only two hydroxyl groups. The fully nitrated derivative (ISDN) was developed first, and the mononitrate (ISMN) was found to be its active metabolite. When administered sublingually, ISDN acts in 2–5 minutes and lasts 1–2 hours. After oral administration of conventional-release tablets, its onset is less than one hour and its duration 4–6 hours. ISDN is used mainly in long-term management of angina, formulated in immediate-release or modified-release tablets (19). ISMN has a twenty minute onset and 8–10 hour duration. It does not undergo first-pass hepatic metabolism, and its bioavailability is nearly 100%. It is used, mainly in the form of extended-release tablets, for the prevention of angina attacks.
Nitrates In Race-Specific Medicine
Whereas the erythritol and mannitol nitrates have fallen into disuse, ISDN has recently attained new prominence and become the subject of controversy. In recent years, epidemiologic studies of CV disease incidence and clinical studies of CV drugs have pointed to some differences between Americans of different races. African-Americans seem more prone than white Americans to develop hypertension and CV disease, including congestive heart failure (CHF). In clinical trials, the beta-blocker carvedilol was equally effective in both black and non-black heart failure patients, whereas the angiotensin-converting enzyme (ACE) inhibitor enalapril was significantly less effective in black patients.
One firm undertook to design a “race-specific” therapy. BiDil®, a combination of an old antihypertensive, hydralazine (37.5 mg), with the NO donor ISDN (20 mg), was tested in a large placebo-controlled study limited to blacks. The African-American Heart Failure Trial (A-HeFT) enrolled more than 1000 patients and the product was so effective in reducing death from CHF, that the study was terminated early. The product received FDA approval in June 2005 for a carefully-worded indication “...treatment of heart failure as an adjunct to standard therapy in self-identified black patients to improve survival ...etc.” (20, 21).
Reflecting Americans’ discomfort at emphasizing racial differences, BiDil® and the A-HeFT study have been criticized. There is concern that future drug development may be fragmented racially rather than being geared toward the entire population. These are important issues that the medical research community will have to resolve.
To Viagra® And Beyond
We have seen that nitric oxide, as generated in vivo from various nitrites and nitrates, was harnessed long ago for the treatment of cardiovascular ailments. Once NO’s action on vascular smooth muscle was elucidated through the work of Furchgott, Ignarro, and Murad, it was natural to ask if NO gas could be utilized for medicinal purposes. The considerable work done on inhaled NO therapy in adults was recently reviewed by Griffiths and Evans (22). NO is generally administered to patients on mechanical ventilation, and may also be given through a nasal cannula or a face mask. Though clinical results have been mixed, Griffiths and Evans concluded that: 1) NO is not effective in patients with acute lung injury or acute respiratory distress syndrome (ARDS), and 2) NO may be useful as a short-term adjunct to cardiorespiratory support in patients with acute hypoxemia, life-threatening pulmonary hypertension, or both.
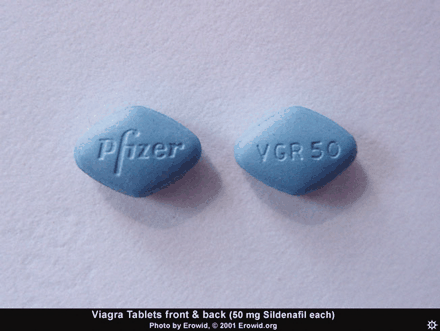
Although the direct administration of NO gas is still problematic, striking therapeutic (and commercial) success has been achieved by enhancing the action of endogenous NO. The first such agent, Viagra® (sildenafil), was synthesized in a search for antihypertensives. Its effect on high blood pressure was mediocre, but it found its niche in the hitherto under-served area of erectile problems.
The physiology of penile erection can be briefly outlined as follows:
-
NO is released in the corpus cavernosum during sexual stimulation.
-
NO activates the enzyme guanylate cyclase.
-
Guanylate cyclase produces increased concentrations of (cGMP).
-
cGMP causes smooth muscle relaxation in corpus cavernosum, allowing influx of blood.
-
cGMP is inactivated by phosphodiesterase type 5 (PDE5).
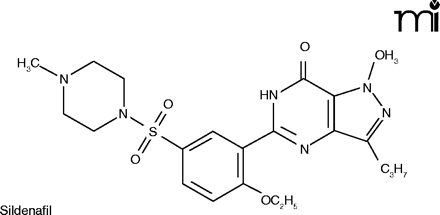
Sildenafil enhances the effect of NO by inhibiting PDE5, slowing down the degradation of cGMP in the corpus cavernosum (23).
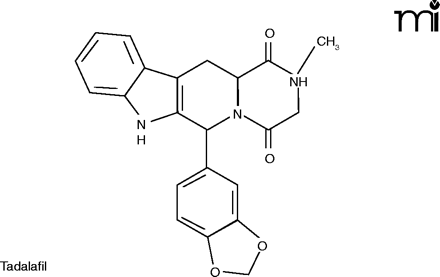
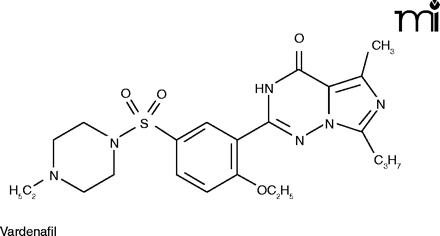
The mention of anything which affects sexual activity tends to evoke ribaldry, but erectile dysfunction (ED) is a serious issue for millions. Besides advancing age, risk factors for ED include diabetes, hypertension, heart disease, and depression (24). The PDE5 inhibitors––Viagra®, Cialis® (tadalafil), and Levitra® (vardenafil)––have proved a boon for men sexually disabled by ED, and indeed for the frustration experienced by their wives as well.
A condition which, unlike ED, does not elicit knowing winks and chuckles, is pulmonary arterial hypertension (PAH). The term refers to a group of diseases in which pulmonary vascular resistance increases inexorably, leading to right ventricular failure and premature death. Treatment includes the use of oxygen, vasodilators, and cardiac medicines such as anticoagulants, digoxin, and diuretics. Approved specifically to treat this condition are three prostanoids and an endothelin-receptor antagonist. These four drugs all present problems with drug delivery, safety, and tolerability (25). Altogether, current treatment of PAH is unsatisfactory.
The vasodilating effects of NO in the lung occur by the same mechanism as described above for the penis. Sildenafil has been suggested as possibly useful in treating PAH, and under the name Sildenafil Use in Pulmonary Arterial Hypertension (SUPER), a 12-week double-blind, placebo-controlled clinical trial was conducted. Three doses of sildenafil––20, 40, and 80mg––were compared against placebo. Sildenafil produced a significant improvement in exercise capacity (the primary endpoint), as measured by the change from baseline to week 12 in the distance walked in six minutes. There was also improvement in WHO (World Health Organization) functional class (Box 1) and hemodynamics, but no improvement in dyspnea (25). The results were not spectacular, but given the paucity of effective treatments for PAH, they were good enough to gain FDA marketing approval. The manufacturer of Viagra® has marketed sildenafil in 20 mg tablets (a lower dosage than that used for ED) specifically for the treatment of PAH. A different trade name, Revatio®, was assigned, to disassociate the product from Viagra® and its implications in the public mind.
World Health Organization (WHO) Classification of Functional Status Regarding Pulmonary Hypertension
The WHO functional classification is a non-invasive means of quantitating exertional intolerance. It is used in evaluating the symptoms of patients being screened or treated for pulmonary hypertension. There are four classes (I through IV, arranged by increasing severity or patient debilitation) that assess a patient’s exercise capacity and comfortable activity level (functional capacity) and establishes a baseline measure for evaluating the response to therapy. A more detailed description may be found in Barst et al. (27).
Looking Forward With NO
Practical applications of the NO discovery have already been realized in the efforts to use the gas itself for various respiratory conditions, in the three approved PDE5 inhibitors (which relieve erectile dysfunction by prolonging NO’s action), and in the use of a PDE5 inhibitor to treat pulmonary hypertension. These developments involve two body areas: the respiratory and the male genitourinary tracts. But NO plays a broader role in the body. Besides its importance in blood vessel relaxation, it functions in the brain and the peripheral autonomic system, acts as a regulator of macrophage activity, and participates in certain organs as well.
The stomach is one such organ where locally generated NO contributes to mucosal defense by attenuating prostaglandin synthesis and by increasing bicarbonate secretion and mucus production. A recently characterized covalent compound consisting of an NO donor, a spacer moiety, and aspirin might allow aspirin to exert its beneficial effects while protecting the gastric mucosa from attack (26).
Undoubtedly, NO research is proceeding along many other fronts. We cannot know which approach will bear fruit, but we can confidently expect further advances in the next several years.
Acknowledgments
The author wishes to thank librarians at Arcadia University, Temple University School of Dentistry, and University of the Sciences in Philadelphia for their helpfulness and perseverance in tracking down needed references.
- © American Society for Pharmacology and Experimental Theraputics 2006
References

Stanley Scheindlin, DSc, RPh, holds graduate degrees in pharmaceutical chemistry and worked in drug product development and regulatory affairs. Now retired, he is a part-time consultant and writes freelance articles for pharmacy-related specialty publications.