
- Institution: Stanford Univ Med Ctr Lane Med Lib/Periodical Dept/Rm L109
- Sign In as Member / Individual
Advanced Drug Delivery Systems That Target The Vascular Endothelium
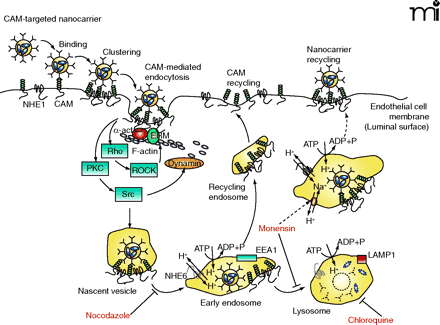
Intracellular drug delivery into vascular endothelial cells using CAM-specific DDS. Multivalent CAM-specific DDS bind to and cluster cell adhesion molecules (CAMs). This action activates protein kinase C (PKC), Src (or other protein tyrosine kinases), and Rho-dependent kinase (ROCK), which regulate the activity of the GTPase dynamin and the amiloride-sensitive sodium/proton exchanger NHE1, all of which induces the formation of actin stress fibers. CAM and NHE1 form a complex, which may help crosslink actin fibers to the CAM cytosolic tail through α-actinin (α-act) and ezrin/radixin/moesin (ERM) family proteins. After internalization, CAM-specific DDS traffic via a microtubule-dependent pathway to endosomes (a process that takes 1–2 hrs) that can be identified by the presence of the protein marker early endosome antigen-1 (EEA1). Endosomes are enriched in NHE6, an intracellular ion exchanger that helps regulate endo-some acidification through the vacuolar H+/ATPase. CAM-specific DDS dissociate from CAM and NHE1 in endosomes and CAM recycles to the plasma membrane. DDS arrive to lysosomes about three hours after their internalization; this process can be identified by colocalization with lysosome-associated membrane protein 1 (LAMP1). In lysosomes, NHE6 becomes inactive, favoring further acidification and degradation of delivered proteins by acidic proteases. Nocodazole (which disrupts the cell microtubule network), chloroquine (a mild base that inhibits lysosomal acidification), and monen-sin (which enhances Na+/H+ exchange in endosomes and induces recycling of CAM-specific DDS to the plasma membrane) can alter the intracellular itinerary of CAM-specific DDS and prolong their effects.


