Emerging concepts from the recent literature
Activation of Cellular Senescence to Combat Tumors?
Our understanding of the biology of cellular senescence remains rudimentary, and consequently we lack pharmacological agents that can affect the process. Rahmouni and coworkers have made the surprising observation that the somewhat enigmatic vaccinia H1-related phosphatase (VHR) controls cellular senescence by altering Erk and Jnk activities in a cell cycle–dependent manner. The expression of this “atypical” dual-specific phosphatase, which lacks the MAP kinase binding CDC25-homology motif, is apparently regulated during the cell cycle. VHR is most abundant in mitotic cells and declines as cells move through the G1 phase, presumably because of a short half-life. During interphase, VHR is mostly localized in the nucleus and thus removed from other signal transduction participants. Nonetheless, loss of the phosphatase activity causes senescence and prolonged hyperactivation of Erk and Jnk pathways. These experimental results might ultimately lead to a pharmacological approach to inducing senescence in tumor cells, which would be a radical approach to treating cancer. [
] —JS Lazo, U Pittsburgh
A Hidden Agonist within Class B Receptors
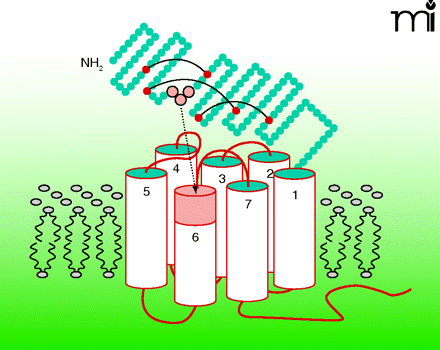
Class-B G protein–coupled receptors (GPCRs) regulate a wide range of endocrine functions and provide attractive therapeutic targets. The mechanisms by which endogenous peptide agonists activate these receptors are not yet fully understood, and synthetic small-molecule drugs to mimic activation have not been found. In the July issue of Mol. Pharmacol., Dong et al. propose a novel paradigm for ligand-induced activation of class-B GPCRs. Based primarily on studies with the secretin receptor, a prototype class-B family member, they provide evidence that the cognate peptide hormone (secretin) exposes a hidden, built-in agonist epitope within the amino terminus of its target GPCR. Oligopeptides corresponding to this receptor epitope (unrelated to secretin) act as full agonists, and can be minimized to tripeptide molecules while conserving biological activity. These findings challenge the current view that the amino termini of peptide hormones per se activate class-B GPCRs and may lead to strategies for defining small-molecule drugs. [
] —M Beinborn, Tufts–New England Med Center
Water-Friendly Cannabinoids
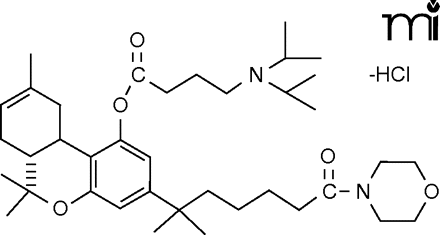
In an upcoming paper in JPET, Martin et al. describe the development of new, valuable tools that are likely to aid the study of endocannabinoid physiology. Currently, experimental studies of cannabinoid receptor agonists require inconvenient solubilization protocols that involve surfactants or other agents. The use of such vehicles is restricted in some tissues, however, and there is concern that the pharmacology could be affected by such administration modalities. Martin and coworkers have now shown that conversion of the tetrahydrocannabinol (THC) phenolic hydroxyl to a morpholinobutyryloxy substituent produces highly potent, water-soluble THC derivatives. Incorporation of nitrogen-containing substituents such as a terminal imidazole into the THC alkyl chain is also shown to increase pharmacological potencies and selectivities for either the CB1 or CB2 receptor. The study introduces new tools for cannabinoid research and contributes new knowledge of the structural determinants for ligand interactions with the two receptor subtypes. [
] —C Beeson, Med U South Carolina
Rheumatoid Arthritis: Painful Messages
With more than two million Americans afflicted, the molecular pathogenesis of rheumatoid arthritis (RA) remains an ongoing area of drug research. Pro-inflammatory cytokines, such as various interleukins (ILs) and tumor necrosis factor alpha (TNF-α), have been implicated in RA and have become important targets for drug development. In a recent posting from Proc. Natl. Acad. Sci. USA, Verri et al. describe their investigation of IL-15, a mediator of RA, and specifically, as the authors now report, of the hyperalgesia that is a symptom of the disease. Verri et al. show that injection of IL-15 into wild-type mice induces hypernociception, and moreover, that endogenous IL-15 is essential to ovalbumin-induced hypernociception. Through a judicious combination of knockout mice, receptor antagonists, and molecular biology, the investigators further establish that three additional players in RA (none of which is TNF-α) form a signaling cascade that culminates in hypernociception: IL-15 → interferon-γ→endothelin-1→prostaglandin E2. [
] —HB Smith, ASPET
- © American Society for Pharmacology and Experimental Theraputics 2006



