Emerging concepts from the recent literature
Tuning the TEMPO of Mitochondrial Harmony
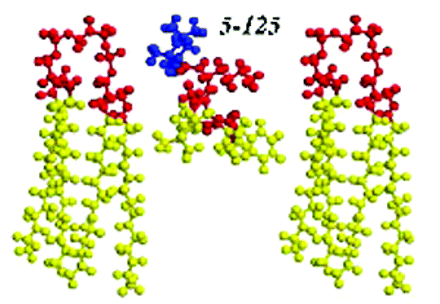
The underlying causative agents in several human cardiovascular and neurodegenerative diseases are though to be reactive oxygen species such as superoxide and hydrogen peroxide, which are produced by mitochondrial respiration. Although antioxidants could presumably ameliorate the pathologies associated with subsequent oxidative damage, the success of such agents has been poor. A number of groups have recently developed antioxidants that specifically target the mitochondria with the hope of increasing therapeutic potential. However, it appears that simply targeting the mitochondria is not sufficient to improve efficacy, and an article appearing in the March 2007 print edition of JPET offers at least one route for further improvement. In the study described by Jiang et al., structural variants of the nitroxide spin label 2,2,6,6-tetramethylpiper-idinyloxy (TEMPO) conjugated to membrane-active gramicidin S peptide fragments were used to define structure activity relationships. Because the nitroxide radicals can accept an electron to give a hydroxyl amine that is then recycled by other enzymes, the nitroxides exhibit superoxide dismutase–like activity. Mitochondrial membrane targeting of the nitroxide-gramicidin conjugates correlated positively with inhibition of apoptosis, but had no similar correlation with simple molecular physicochemical parameters such as theoretical lipophilicity or octanol-water partition coefficients. Both NMR and CD spectroscopic studies of the most potent molecules suggested that preservation of a peptide β-turn domain in the gramicidin-derived peptide fragment was important for activity. Extensive molecular dynamics calculations of the molecules at a lipid membrane surface further suggested that positioning of the agent such that the nitroxide is poised at the membrane apical polar surface correlates best with antiapoptotic activity and efficacy. These results suggest that both organelle targeting and the positioning within the organelle will be critical for the design of effective mitochondrial antioxidant therapeutics. [Jiang, J., Kurnikov, I., Belikova, N.A. et al. Structural requirements for optimized delivery, inhibition of oxidative stress and anti-apoptotic activity of targeted nitroxides. J. Pharmacol. Exp. Ther. Epub ahead of print; doi: 10.1124/jpet.106.114769v1 (2006).]—C Beeson, Medical University of South Carolina
First-in-class: RGS Protein Inhibitor
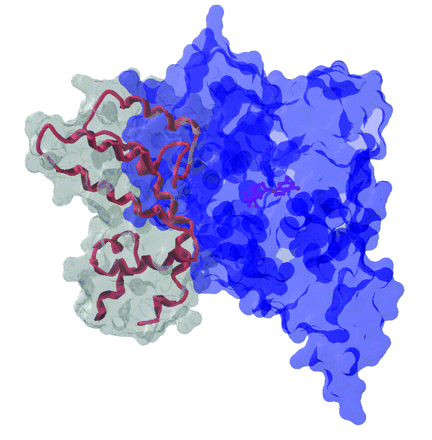
Perturbation of G protein–coupled receptor pathways is implicated in many pathologies ranging from addiction to hypertension. Regulators of G-protein signaling (RGS proteins) serve as GTPase-accelerating proteins (GAPs), promoting the hydrolysis of GTP to GDP and returning GPCR signaling to basal levels. Unique tissue distributions and receptor-selective effects of particular RGS proteins have led to speculation that a small molecule inhibitor of RGS protein GAP activity would be a valuable addition to our pharmacologic armamentarium. In the January issue of Mol. Pharmacol., Roman et al. describe a small molecule (CCG-4986) identified in a flow-cytometric protein interaction assay as an inhibitor of RGS4/Gαo binding. CCG-4986 inhibits the GAP activity of RGS4 in single-turnover GTP hydrolysis and quasi-cellular assays, but has no effect on RGS8. Along with an earlier C. elegans genetics study from Fitzgerald et al. in PLoS Genet., this paper provides one of the first examples of chemical inhibition of a subset of RGS proteins. [Roman, D.L., Talbot, J.N., Roof, R.A., Sunahara, R.K., Traynor, J.R., and Neubig, R.R. Identification of small-molecule inhibitors of RGS4 using a high-throughput flow cytometry protein interaction assay. Mol. Pharmacol. Epub ahead of print; doi: 10.1124/mol.106.028670 (2006).] —AJ Kimple and D.P. Siderovski, UNC–Chapel Hill
Hsp90 Function: Bringing Cancer, Neurological Disease, and Depression into the Fold
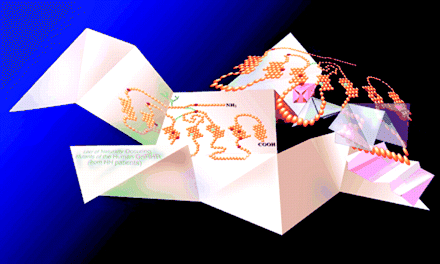
Our understanding of the mechanisms regulating the desensitization of G-protein coupled receptor-mediated responses has been enhanced by the recent recognition of the important role of G-protein coupled receptor kinase (GRK) proteins. Modulation of GRK expression and activity is an attractive target for therapeutic manipulation of numerous signaling pathways. For example, GRK3 mediates inter-nalization of the α2B-adrenoceptor, following stimulation. The March issue of JPET includes an article on the role of the chaperone protein Hsp90 in regulating neuronal GRK3 expression, following stimulation with epinephrine. A polymorphism in GRK3 is associated with bipolar disorder; the severity of disease is inversely proportional to GRK3 expression in patient lymphocytes. Other workers have shown that the Hsp90 chaperone protein is required for expression of the related GRK2 protein via either protein stabilization or protection from proteasomal degradation. In the present study, Hsp90 associated with GRK3 and disruption of this binding led to proteasomal degradation of GRK3. Agonist activation of the α2B-adrenoceptor with epinephrine led to increased Hsp90 expression associated with GRK3 and calcium-mediated activation of the cysteine protease calpain significantly decreased GRK3 expression. A role for Hsp90 has been implicated in several neurodegenerative diseases (e.g., Parkinson, Huntington, and Alzheimer Diseases) that share the trait of dysregulated protein folding and accumulation of aggregates. The observations reported in this study, along with others, further support the emerging concept of Hsp90 as a regulator of signaling processes, suggesting that it also could be a therapeutic target akin to GRK proteins for the treatment of multiple disease states. [Salim, S. and Eikenburg, D.C. Role of Hsp90 and protein degradation in regulating neuronal levels of GRK3. J. Pharmacol. Exp. Ther. Epub ahead of print; doi: 10.1124/jpet.jpet.106.114835v1 (2006).] —J Isaacs, Medical University of South Carolina
- © American Society for Pharmacology and Experimental Theraputics 2007



