Inhibiting Sperm Motility for Male Contraception: Will the Sperm Tail Be its “Achilles Heel”?
Research attempting to develop a contraceptive for men has been ongoing since the introduction of the estrogen-progesterone “pill” for women. Progress has been made in both hormonal and non-hormonal approaches; however, a safe and effective approach has remained elusive. In part, this is because of the ability of the testes to produce an extraordinary number of sperm. In contrast to the single oocyte produced monthly by the ovaries, a man’s testes produce roughly 1,000 sperm per second during adult life (1). Because pregnancy has been reported in experimental contraceptive trials even when spermatogenesis is suppressed to less than 5% of normal levels (2), an effective male contraceptive must almost completely inhibit sperm production or function. In addition, the developing sperm are protected by the “blood-testis barrier,” which can prevent drugs intended to block sperm production from reaching the developing sperm (3). Indeed, the challenges posed by male contraceptive development are so great that some are skeptical that a male contraceptive will ever be found (4).
To date, most male contraceptive research has focused on the hormonal approach that has proved so successful for female contraception. When the hormone testosterone is administered to a man, it functions as a contraceptive by suppressing the secretion of luteinizing hormone (LH) and follicle stimulating hormone (FSH) from the pituitary gland, thereby depriving the testis of the signals required for spermatogenesis. After two to three months of treatment, the ensuing low concentrations of LH and FSH lead to markedly decreased sperm counts and effective contraception in a majority of men. However, spermatogenesis is not completely suppressed in all men, meaning that some potential for fertility persists. The addition of progestins can enhance the suppression of spermatogenesis by further inhibiting the release of FSH and LH, but even in the best of circumstances, roughly 10% of men retain some potential for fertility (5). As a result, no hormonal method has been approved by regulatory agencies for commercial introduction, and the interest of pharmaceutical companies appears to be shifting to non-hormonal approaches.
Non-hormonal approaches to male contraceptive development can either target sperm production (e.g., gossypol) or seek to impair the function of ejaculated sperm. Recently, several investigators have focused on inhibition of sperm motility as a promising target for male contraceptive development (Figure 1⇓). Sperm become motile by initiating flagellar beating during ejaculation in order to swim up the female reproductive tract. Immediately upon ejaculation, a sperm can locomote between 1–3 millimeters per minute by whipping its flagellum three-dimensionally in an elliptical cone (6). Prior to fertilization, sperm dramatically increase the frequency and force of their flagellar beating, a process called “hyperactivation,” in order to reach the ovum and to penetrate its cumulus and the zona pellucida. Blocking sperm motility, hyperactivation, or both has great appeal as a male contraceptive. A drug targeting motility or hyperactivation would not necessarily have to be able to cross the “blood-testes” barrier as long as it partitioned into seminal fluid and was ejaculated with the sperm. In addition, a drug working on blocking sperm motility might have a very rapid onset of action, possibly allowing for administration immediately prior to intercourse.
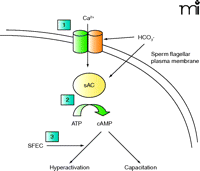
One approach to blocking sperm motility––and sperm hyper-activation in particular––has focused on a group of four novel sperm transmembrane calcium channels called “CatSpers,” first described in 2001 (7). These transmembrane proteins allow for Ca2+ entry into the sperm tail through a bicarbonate-activated, voltage-sensitive channel. The rise in intracellular calcium mediated by the CatSpers is directly responsible for the increase in flagellar beat frequency that characterizes sperm hyperactivation. As a result, CatSpers are critical for fertility, and CatSper-knockout animals are infertile (8). Thus, CatSpers are promising targets for male contraceptive development because they are highly specific for sperm, although no antagonists of these channels have been reported in the literature to date.
Additional promising targets for inhibiting sperm motility are the proteins of the principal piece of the flagellum (Figure 2⇓). In one recent report, mass spectrometry proteomic analysis performed on isolated human fibrous sheaths was used to identify a several proteins that co-localize, as observed by immunofluorescence, to the fibrous sheath of the flagellum (9). Most of the isolated proteins were known glycolytic enzymes, but the authors also identified a unique adenine nucleotide translocase. This protein, called sperm flagellar energy carrier [SFEC, also termed ADP-ATP carrier protein 4 (AAC4)] by the investigators, appears to participate in the transport of ATP within the principal piece. The authors hypothesize that SFEC shuttles ATP to the core of the flagellar axoneme, where dynein ATPases allow the microtubules of the flagellum to slide across one another and generate flagellar movement. Given the absence of mitochondria in the principal piece and the requirement for energy production in the low oxygen tension present in the female reproductive tract where flagellar action is activated the reliance on glycolysis to fuel flagellar movement seems plausible. Lastly, SFEC possesses a unique protein motif at the entrance to the nucleotide-binding pocket, as suggested by structural modeling, that might be an attractive target for drug targeting.
A final intriguing target for male contraceptive motility research is the unique “soluble” adenylate cyclase (sAC) found in sperm. This cyclase is termed “soluble” because it lacks a transmembrane anchor. sAC produces adenosine 3′,5′-monophosphate [cyclic AMP, (cAMP)] in the cytoplasm of the sperm, and its activity is potentiated by bicarbonate and by calcium, known triggers of sperm hyper-activation (10). In addition, increased intracellular cAMP is essential for preparing the sperm for fertilization, a process known as “capacitation.” Therefore, an inhibitor of sAC may utilize two mechanisms to impair fertility: 1) inhibiting sperm motility and hyper-activation, and 2) impairing capacitation. Mice lacking sAC are infertile and their sperm are immotile (11). Furthermore, recent work with KH7, a specific inhibitor of sAC, revealed that sAC inhibition markedly impaired the ability of mouse sperm to undergo capacitation, achieve a hyperactivated state and, ultimately, fertilize oocytes in vitro (12). Future work on this inhibition of sAC as a potential contraceptive motility target in sperm is clearly warranted.
The challenges inherent in male contraceptive development are indeed daunting. Thankfully, many investigators remain interested in the identification of novel targets for male contraceptive development. Recently, sperm motility has become an intriguing focus of such efforts; however, only time will tell if the sperm tail proves to be its “Achilles heel” for contraceptive purposes. It is hoped that investigation into this and other approaches to blocking sperm production and function will bring the dream of the “male pill” to fruition. doi:10.1124/mi.7.2.5
Processes involved in sperm motility, hyperactivation, and capacitation, and potential contraceptive targets. In normal sperm, these processes are mediated by an influx of calcium and bicarbonate encountered in the female reproductive tract. These processes are driven by increased intracellular cAMP. Potential contraceptive motility targets could include: 1) “CatSper” transmembrane calcium channels (two of four shown); 2) “soluble” adenylyl cyclase; and 3) SFEC, the inhibition of which could block the delivery of ATP necessary to generate flagellar activity. HCO3−, bicarbonate ion; sAC, soluble adenylyl cyclase; SFEC, sperm flagellar energy carrier protein.
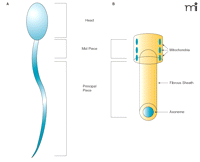
Structure of sperm. A. Major elements of human sperm. B. The ultrastructure of the mid piece (upper) and the principal piece (lower). Note the absence of mitochondria in the principal piece––the main site of flagellar activity.
- © American Society for Pharmacology and Experimental Theraputics 2007
References

John K. Amory, MD, MPH, is an Associate Professor in the Department of Medicine at the University of Washington School of Medicine He earned his MD at the University of California–San Francisco (UCSF) Medical School and undertook a residency in internal medicine at UCSF. His research interests include male contraception and infertility, testosterone replacement therapy, the effects of androgens in aging, and the study of Klinefelter Syndrome. E-mail jamory{at}u.washington.edu; fax (206) 616-5365.