Optimizing Combination Chemotherapy by Controlling Drug Ratios
Abstract
Cancer chemotherapy treatments typically employ drug combinations in which the dose of each agent is pushed to the brink of unacceptable toxicity; however, emerging evidence indicates that this approach may not be providing optimal efficacy due to the manner in which drugs interact. Specifically, whereas certain ratios of combined drugs can be synergistic, other ratios of the same agents may be antagonistic, implying that the most efficacious combinations may be those that utilize certain agents at reduced doses. Advances in nano-scale drug delivery vehicles now enable the translation of in vitro information on synergistic drug ratios into improved anticancer combination therapies in which the desired drug ratio can be controlled and maintained following administration in vivo, so that synergistic effects can be exploited. This “ratiometric” approach to combination chemotherapy opens new opportunities to enhance the effectiveness of existing and future treatment regimens across a spectrum of human diseases.
Introduction
Drug combinations have played a particularly prominent role in the treatment of cancer. They have been employed widely for over half a century and, with only a few exceptions, are the only means available to cure metastatic disease. During the past two decades, significant advancements have been made in understanding the molecular basis for the initiation, growth, and metastasis of human malignancies. This knowledge has fuelled an expanded effort to rationally develop combination therapeutics that could significantly improve patient outcomes in cancer (1, 2). In addition, as the multiple molecular changes causing noncancerous disease, including infectious diseases [e.g., Human Immunodeficiency Virus (HIV)] and cardiovascular disorders, have continued to be elucidated, the use of therapeutic drug combinations has found widening relevance.
Despite these advances, the fundamentals of combination chemotherapy development have remained largely unchanged from those originally pioneered by Frei and coworkers in the 1960s (3). The general principle has been to: 1) use drugs with non-overlapping toxicities so that each drug in the combination can be administered at near-maximal dose; 2) combine agents with different mechanisms of action in order to inhibit the emergence of broad-spectrum drug resistance; and 3) administer the full combination as early as possible in the disease (3, 4). Occasionally, clinical trials have investigated the scheduling or sequencing of drugs in a combination, but even in these studies the focus has remained to use every agent at its maximum tolerated dose (MTD). The principle underlying this approach is that maximal therapeutic activity will be achieved by co-administering each drug at its MTD. In this way, the administration of multiple drugs together follows the conventional wisdom that “more is better.”
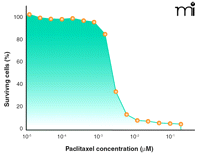
In order to understand the rationale for utilizing anticancer drugs at MTD, it is instructive to review the dose response relationship typically observed for these agents. Figure 1⇓ presents the percent inhibition of growth of A2780 human ovarian cancer cells exposed to various concentrations of paclitaxel in vitro. Below a concentration of 1 nM, virtually no antitumor activity is obtained. Increasing the paclitaxel concentration from 1nM to 10 nM results in a dramatic inflection of antitumor activity, such that cell growth inhibition increases from below 10% to greater than 90%. In fact, a semilogarithmic plot of cell survival as a function of drug concentration is sigmoidal for most anticancer agents, and the therapeutic window is typically narrow (minimum therapeutic doses are often within two- to fivefold of MTD). Therefore, it is believed that administering drugs at an MTD maximizes exposure of cells to drug concentrations that elicit maximum tumor growth inhibition.
A common cytotoxicity analysis in cancer pharmacology: The dose-response curve. Shown here is the antitumor activity of paclitaxel administered to A2780 human ovarian cancer cells for 72h in vitro.
Although the “more-is-better” approach to anticancer combination chemotherapy is empirically rational, clinical data have suggested that this strategy may in fact fail to exploit the full therapeutic potential of many drug combinations. For example, a meta-analysis of sixty-five trials (13,601 patients) that assessed the benefits of adding a drug to single- or dual-agent chemotherapy regimens in advanced non-small cell lung cancer (NSCLC) revealed a statistically significant increase in tumor response rates with the additional agent; however, the additional agent did not provide a survival advantage (5). In fact, examination of thirty-three randomized phase III trials of NSCLC initiated in North America between 1973 and 1994 revealed that only five (15%) treatment regimes showed a statistically significant, albeit modest (i.e., on the order of two months), prolongation of survival between treatment arms (6).
There are undoubtedly several molecular and pharmacological factors that determine the effectiveness of drug combinations; however, the “more-is-better” philosophy for anticancer combinations may not always be therapeutically optimal because of the manner in which anticancer drugs interact. Emerging evidence is revealing that certain ratios of combined agents can be synergistic, whereas other ratios of the same drugs are merely additive or even antagonistic (7, 8). If antagonistic ratios, rather than synergistic ratios, ensue after administration of combinations based solely on MTD, the full benefits of these therapies will go untapped, much to the detriment of cancer patients. In this review we examine the scientific basis of drug ratio–dependent synergy and consider its implications on existing combination chemotherapies. The clinical exploitation of synergistic drug ratios through the use of drug delivery vehicles is also explored.
Drug Ratio–Dependent Synergy Revealed in Vitro
Evaluation of synergistic drug combinations is frequently conducted in cell culture, where the concentration and duration of administered drug(s) can be tightly controlled, and the inhibition of tumor cell growth can be readily measured. In these experiments, the manner in which agents are combined can vary significantly. In one common approach, each of two drugs (drug A and drug B) is prepared at a series of concentrations that spans the dose response curve for the given drug (9). Each concentration of drug sample A is mixed with each concentration of drug sample B, thereby producing a matrix of multiple stock admixtures, containing both drugs together in solution at a variety of concentrations and ratios. Any one of a number of available mathematical algorithms can then be used to assess whether each admixture provides more (synergy), equivalent (additive), or less (antagonism) tumor growth inhibition than predicted based on the antitumor activity of the given concentrations of drug A and drug B observed upon their individual administration (10). Unfortunately, this approach yields an ad hoc assortment of drug ratios at a variety of drug concentrations, which complicates the assessment of clinical implications of drug ratio–dependent efficacy.
In 1984, Chou and colleagues developed an approach that involves a median effect analysis for evaluating synergy (11). This method typically combines two drugs at a fixed ratio based on the relative concentrations of the individual agents that elicit 50%-inhibition of cell growth inhibition (i.e., based on the IC50 values of the combined drugs). The tumor growth inhibition obtained for this fixed ratio combination over a range of concentrations is compared to that obtained for the individual drugs, and a measure of the synergy between the two drugs, referred to as the combination index (CI), can be calculated using a median-effect mathematical algorithm. A drug combination is synergistic if its CI value is significantly below 1 (CI < 0.9); the combination is additive where the CI is between 0.9 and 1.1; and the combination is antagonistic as indicated by CI values above 1.1. The median effect analysis approach has been refined in practice over the years to yield user-friendly software programs that are widely employed in drug combination research (12). Although combination analyses have historically been investigated based on relative IC50 values of the two drugs being combined, when one carefully examines published in vitro drug combination data, indications of drug ratio–dependent synergy can be extracted (13–17). Several examples exist for a variety of cancer drug classes, including taxanes, camptothecins, anthracyclines, vinca alkaloids, antimetabolites, and platinum agents, where certain drug ratios are synergistic and others are antagonistic.
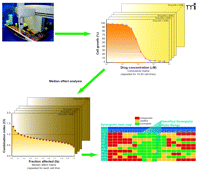
Ideally, a systematic evaluation of drug combinations should examine a series of fixed drug ratios, with each fixed ratio diluted over a range of concentrations that elicits a broad spectrum of the biological response [e.g., ranging from less than 10% to greater than 90% inhibition of tumor cell growth; see references (7) and (8)]. A systematic evaluation of this type is shown in Figure 2⇓, where we use high-throughput screening to generate tumor growth inhibition curves for a number of drug ratios in multiple tumor cell lines. These cytotoxicity curves are analyzed by the median effect method to yield synergy plots of CI as a function of the fraction of cells affected (fa). We then examine the CI values at high tumor growth inhibition (which is most relevant for cancer treatment) and create a color-coded “synergy heat map” that vividly highlights the synergy, additivity, or antagonism of the different drug ratios exposed to the various tumor cell lines of interest.
Systematic in vitro identification of synergistic drug interactions. Automated assays allow for a wide variety of drug ratios to be tested on multiple cell lines. Mathematical analysis reveals the presence of common trends in drug ratio–dependent synergy (green, synergy; yellow, additivity; red, antagonism). See text for details.
This approach is capable of revealing common trends in drug ratio dependency among different tumor types, and we have found that many commonly used drug combinations, representing a wide range of drug classes with disparate chemical and functional properties, exhibit striking drug ratio-dependent synergy. Certain ratios consistently display synergy across a panel of tumor cell types, whereas other ratios can be strongly antagonistic (7, 8), which suggests that the phenomenon of drug ratio-dependent tumor cell growth inhibition is mechanistically unbiased and of widespread importance to combination chemotherapy. Thus, it would appear that certain anticancer drug ratios trigger a conflation of pro-apoptotic signals that lead to an amplification of cell death (synergy), whereas other ratios lead to the activation of pro-survival cellular responses (antagonism). These responses likely reflect the significant crosstalk that transpires among various biochemical pathways. Also, factors continue to be identified that influence both pro-apoptotic and proliferative responses, the balance of which depends both on microenvironmental conditions and the coordination of various signaling molecules (18, 19).
Exploiting Drug Ratio–Dependent Informatics: Nano-Scale Delivery Vehicles In Vivo
When considered in the context of in vivo drug pharmacology, the exploitation of synergistic drug ratio “windows” and the avoidance of antagonistic interactions will require the control of drug ratios after systemic administration. Although we can determine the relationship between synergy/antagonism and drug:drug ratio for a given combination in vitro, this information in itself is insufficient to achieve improved therapeutic outcomes in vivo because of the differential distribution and metabolism of the individual drugs within the conventionally administered “cocktail.” For example, cisplatin, with an elimination half-life of twenty to forty-five minutes (20), is removed rapidly from plasma, whereas the terminal elimination half-life for paclitaxel is greater than ten hours (21). Given such disparate pharmacokinetics, it is clear that when a combination of these two drugs is administered intravenously for the treatment of NSCLC using conventional aqueous formulations, the ratio of the drugs will undoubtedly change dramatically and rapidly after injection.
The inability to control drug ratios in vivo may partly explain the shortcomings in clinical efficacy seen with many conventional combination chemotherapy regimens. First, it is likely that for many anticancer drug combinations, the drug ratios arising from in vivo doses administered on the basis of MTD will not correspond to the optimal ratio identified in vitro. Perhaps more importantly, even when armed with the knowledge of drug ratio–dependent synergy information, the ability to achieve synergistic ratios in vivo is precluded by the dissimilar pharmacokinetics of the individual agents in conventional chemotherapy cocktails. It is thus difficult to achieve a desired drug ratio in the blood—and it is even more difficult, if not impossible, to control the relative levels of drugs that ultimately penetrate tumor tissue.
Advances in nanotechnology have provided an opportunity to address the problem of controlling drug ratios in vivo. Nano-scale delivery vehicles (20–200nm diameter) such as liposomes, nanoparticles, and polymer–drug conjugates have been used extensively for the delivery of anticancer drugs and other therapeutic agents (22, 23). Liposomes are spherical envelopes of amphipathic lipid, typically phospholipids, surrounding an internal aqueous core. These vehicles are most often utilized to deliver water-soluble drugs, and the composition of the lipid bilayer that forms the envelope can be manipulated in order to regulate the rate of drug release after systemic administration. Liposomes are arguably the most advanced nano-scale drug delivery system available, with several liposome-based products approved for human use in Europe and North America (24). Nanoparticles and polymer conjugates have been used more extensively for the delivery of hydrophobic agents (e.g., paclitaxel). Nanoparticles are of a size similar to liposomes (typically 30–100 nm diameter) but have a solid core composed of hydrophobic polymer excipients in which the hydrophobic drug is co-dispersed. As with liposomes, the rate of drug release can be controlled by varying the excipients in the nanoparticle core. To date, polymer drug conjugates have been employed primarily to increase the aqueous solubility of hydrophobic drugs, such as paclitaxel and camptothecin. In these cases, the parent drugs are coupled to water-soluble polymers (e.g., polyglutamic acid) through chemical linkages that are slowly hydrolyzed in vivo after injection (25, 26).
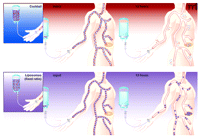
Nano-scale drug delivery vehicles exhibit three features that are particularly well suited for controlling and coordinating the pharmacokinetics of co-administered drugs so that the desired ratio can be maintained in vivo: 1) Drug delivery vehicles of about 100 nm (approximately 100-times smaller than a red blood cell) do not readily escape out of the blood vessels in most healthy tissues. This delays drug elimination from the circulation and eliminates early-phase drug distribution as one source of differing pharmacokinetics between various anticancer drugs. 2) Nano-scale drug delivery vehicles tend to accumulate in tumor tissues through a process referred to as enhanced penetration and retention (22, 23). The leaky vasculature and poor lymphatic drainage associated with sites of tumor growth together produce a “sieving” effect that results in selective drug accumulation at tumor sites. Nano-scale vehicles containing drug combinations are accordingly able to deliver drugs to the tumor at the desired (synergistic) ratio. 3) Finally, the composition of drug delivery vehicles can be manipulated to control the release rate of anticancer agents following injection. Formulation parameters that maximize the therapeutic potency and minimize toxicity of the associated drugs can be engineered to maintain the injected drug ratio for prolonged periods of time post-administration, thus maximizing the therapeutic index (27). Figure 3⇓ illustrates how liposomes and other small particulate delivery vehicles can dramatically alter the way in which drug combinations distribute throughout the body compared to conventional aqueous cocktails. In contrast to the control of drug ratio exhibited by combinations administered inside delivery vehicles, the combination injected as a free drug cocktail rapidly distributes into healthy and tumor tissues at drug ratios that differ greatly from the administered ratio.
The clinical application of drug–drug synergy depends on controlled delivery of the desired ratio to the in vivo target. In the upper panel, a drug cocktail is prepared at the desired ratio, but processes of drug distribution, metabolism, and excretion will act differentially upon the two drugs and cause the ratio to vary subsequent to injection. In this schematic, the two drugs distribute extensively and rapidly into tissues shortly after injection; note that the ratio of drugs that reaches the tumor has been displaced fivefold from the desired [injected (1:1)] ratio. In the lower panel, liposomes that contain the synergistic (1:1) ratio maintain and selectively deliver this drug ratio to the tumor. The appropriately designed drug delivery vehicles maintain the drugs in the blood at much higher concentrations for extended periods of time and, most importantly, at the effective, synergistic ratio. See text for details.
Translating Drug Ratio–Dependent Synergy From Bench to Bedside
Drug delivery science now gives us the ability to control drug ratios after systemic administration and test whether drug ratio–dependent synergy occurs in vivo. Importantly, the relationship between drug ratio and activity can be compared between in vitro and in vivo systems to determine whether delivery technology can be used prospectively to translate drug ratio informatics from cell culture directly into animals.
We have developed and extensively characterized a liposomal formulation (CPX-1, Celator Pharmaceuticals) that encapsulates irinotecan and floxuridine at a 1:1 molar ratio. This combination was selected for study because co-administration of irinotecan and fluorinated pyrimidine is standard treatment for metastatic colorectal cancer (CRC), and previous laboratory investigations have suggested that these two agents can act synergistically under appropriate conditions (28). We constructed “synergy heat maps” that analyzed several human tumor cell lines as schematized in Figure 2⇑ (8, 29) and found the 1:1 ratio of the two drugs to be consistently synergistic, whereas increasing or decreasing this ratio resulted in a loss of synergy and the appearance of significant antagonism. This effect was most pronounced in CRC tumor lines, where the drug–drug interaction shifted from strong synergy to strong antagonism as the irinotecan:floxuridine molar ratio was changed from 1:1 to 10:1.
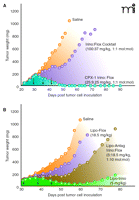
CPX-1 not only maintains the target synergistic ratio of the two drugs (1:1) for up to twenty-four hours in the plasma of mice, but also delivers high equimolar amounts of the two drugs to human solid tumor xenografts, a characteristic that cannot be achieved through administration of a conventional cocktail (8, 29). When tested for efficacy against xenografts of CRC and pancreatic cancer, CPX-1 elicits significant tumor regression, and in some models complete tumor ablation is achieved, whereas the free drug cocktail provides minimal tumor growth inhibition (Figure 4⇓). Importantly, when an antagonistic irinotecan:floxuridine ratio is delivered in a liposome vehicle, the efficacy of this combination is significantly curtailed, clearly indicating that at this ratio, floxuridine inhibits the efficacy of irinotecan in contrast to the in vivo synergy observed for CPX-1. In addition to establishing that desired drug ratios can be maintained in vivo, these results are important in demonstrating that in vitro information about the drug ratio–dependent efficacy of drug combinations can be translated into animals.
Increased efficacy of irinotecan and floxuridine depends on delivery of the synergistic ratio. (A) Liposomal delivery of a 1:1 molar ratio of the two drugs (synergistic in vitro) cures mice bearing human pancreatic solid tumors, whereas the conventional cocktail has minimal activity. (B) Liposomal delivery of a 1:10 molar ratio (antagonistic in vitro) is significantly less active against human pancreatic tumors growing in mice; the delivered 1:10 ratio is, in fact, less active than the administration of liposomal irinotecan alone. In both panels, arrows indicate treatment days. See text for details.
The successful translation of in vitro drug ratio–dependent synergy information for application in vivo has also been demonstrated with other drug combinations. The combination of cytarabine and daunorubicin is standard chemotherapy for acute myelogenous leukemia (AML). We found a 5:1 molar ratio of these two drugs (cytarabine:daunorubicin) to be synergistic in our in vitro system, whereas ratios of 1:5 and 1:10 were antagonistic (8). When these drugs were delivered in vivo using 100-nm liposomes (CPX-351, Celator Pharmaceuticals), the 5:1 ratio was maintained for over twenty-four hours; this formulation, moreover, was able to completely cure leukemia-bearing mice. In contrast, an aqueous cocktail of the two drugs afforded only a modest increase in survival and elicited no long-term cures even though much higher drug doses were administered relative to liposomal delivery (8). Indeed, the more efficacious liposomal delivery system utilized daunorubicin at only one-half of its MTD. Increasing the daunorubicin concentration within liposomes to produce antagonistic ratios predecitably resulted in formulations less effective thatn CPX-351.
Another combination that illustrates the advantages of controlling drug ratios is provided by cisplatin and topotecan, which we identified as synergistic at a 10:1 molar ratio when administered to human NSCLC cells in vitro. In a liposomal formulation, we found that this ratio could be maintained in the blood for up to twenty-four hours and thereby block the growth of human lung cancer solid tumors in immune compromised mice; the corresponding conventional drug cocktail achieved little antitumor activity (7). The improved efficacy observed for the fixed synergistic ratio formulation is striking, given the fact that both cisplatin and topotecan administered individually as liposome-encapsulated formulations were virtually inactive against this tumor model. Notably, the dose of topotecan delivered in combination with cisplatin by the liposomal formulation was approximately 10-fold lower than its MTD.
More recently, CPX-1 has proven capable of maintaining the desired 1:1 ratio of irinotecan and floxuridine and eliciting significant antitumor activity in a Phase I trial involving highly pre-treated cancer patients (30, 31). These results provide encouraging signs that combination chemotherapy can be optimized by controlling drug ratios. A Phase I trial of CPX-351 is also ongoing. The clinical impact of nano-scale drug delivery systems may be very wide, enabling drug ratio–dependent synergy relationships established in vitro to be successfully brought to patients. This ratiometric approach may also find application across a wide range of conventional and genome-based combination therapeutics, thereby greatly expanding our ability to treat complex diseases.
Conclusion
The systematic analysis of drug combinations in vitro can establish synergistic relationships that had previously gone unrecognized in the treatment of cancer and other diseases. The fact that some drug ratios can be highly antagonistic implies that existing aqueous-based drug combinations may be significantly less efficacious than presumed due to the dissimilar pharmacokenetics of individual agents. The ability to control drug ratios in vivo through the use of nano-scale drug delivery vehicles provides the opportunity to improve drug efficacy. Preclinical and early clinical evidence obtained for synergistic, fixed-ratio anticancer drug combinations delivered in liposomes indicates that the systematic evaluation of drug combinations in vitro can be translated into meaningful improvements in clinical therapies. Furthermore, this approach has the potential to be applied to numerous other illnesses in which multiple interacting mechanisms are responsible for disease progression or response to therapeutic interventions.
- © American Society for Pharmacology and Experimental Theraputics 2007
References

Andrew S. Janoff, PhD, is CEO of Celator Pharmaceuticals. He has led research and development efforts for several novel drugs and drug delivery technologies. Previously, he was a vice president of research and development at Elan Corporation as well as vice president and a scientific founder of The Liposome Company, Inc.

Lawrence Mayer, PhD, is President & Head of Research at Celator Pharmaceuticals. His interests span anticancer therapeutics and the application of novel drug delivery systems to enhance their efficacy He is also adjunct professor in the Faculty of Pharmaceutical Sciences at the University of British Columbia. E-mail lmayer{at}celatorpharma.com; fax 604-708-5883.