Emerging concepts from the recent literature
Psychotic Receptors: mGlu2 and 5-HT2A
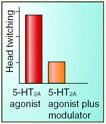
Psychotomimetic drugs (e.g., hallucinogens such as LSD) are commonly used in rodents to generate symptoms of acute schizophrenia. Recently, the central role of serotonin 5-HT2A receptors in mediating the psychotropic actions of hallucinogens has been firmly established. Specifically, 5-HT2A receptors increase glutamatergic synaptic activity within the pyramidal cells of the prefrontal cortex (PFC). The feasibility of therapeutically manipulating this circuitry awaits further study. Nevertheless, it is clear that subtype-2 metabotropic glutamate (mGlu2) receptors inhibit the actions of hallucinogens and prevent the glutamatergic synaptic activity that ensues from activation of 5-HT2A receptors. Clinical studies indicate that selective mGlu2 receptor agonists are effective in treating the symptoms of schizophrenic patients. Benneyworth et al. now report, in a recent issue of Mol. Pharmacol., that biphenyl-indanone A, a positive allosteric modulator selective for mGlu2 receptors, modulates excitatory postsynaptic currents in the PFC, attenuates the in vivo agonism of 5-HT2A receptors, and mitigates hallucinogen-induced head twitch behavior. These data corroborate pharmacological strategies for targeting mGlu2 receptors in the treatment of schizophrenia. [
] —J Bockaert, CNRS, France
Adenosine and Angiogenesis: Entering the Epac Epoch?
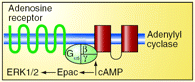
Adenosine generated in tissues during ischemia promotes the growth of new blood vessels. Proliferation of vascular endothelial cells is required for this angiogenic response. Like many G protein–coupled receptors, endothelial adenosine receptors elevate intracellular concentrations of cAMP, a second messenger that is now known to activate multiple effectors. For example, a guanine nucleotide exchange factor for small GTPases has been named in recognition of its activation by cAMP: Epac (i.e., exchange protein activated by cAMP). In the upcoming September issue of JPET, Fang and Olah provide evidence that the angiogenic response is mediated via activation of endothelial cell adenosine receptors that stimulate ERK1/2 through Epac signaling. Specifically, the authors report that ERK1/2 activation by an adenosine receptor agonist can be enhanced by overexpression of Epac, whereas reductions in Epac expression can prevent the adenosine receptor–mediated activation of ERK1/2. Although it is not yet proven that Epac activates ERK1/2 to control endothelial cell proliferation, several reports suggest a role for Epac in the regulation of endothelial permeability. Together, these results may bring attention to Epac as a new target in the treatment of pathologies that involve angiogenesis. [Fang, Y. and Olah, M.E. Cyclic AMP-dependent, protein kinase A–independent activation of extracellular signal–regulated kinase 1/2 (ERK 1/2) following adenosine receptor stimulation in human umbilical vein endothelial cells—Role of Epac1. J. Pharmacol. Exp. Ther. Epub ahead of print; doi: 10.1124/jpet.107.119933 (2007).] —C Beeson, Medical University of South Carolina
Synthetic Lethal Screening Identifies Drug Synergies
Current chemotherapy suffers from a slim therapeutic index, with patients picking their poison: side effects and toxicity from effective drug doses, or tumor recurrence at low drug doses. The synergy of two drugs working together, however, can result in sufficient anticancer potency at non-toxic doses. But identifying such synergistic interactions has been a slow process. Now, Whitehurst et al. describe an unbiased, genome-wide siRNA synthetic-lethal screen to identify biomolecules that act to limit cancer cell susceptibility to paclitaxel. The authors employ a high-throughput cell-based 84,508-siRNA screen to isolate a high-confidence list of 87 genes that, when silenced, drastically enhance (e.g., 1000-fold) the chemosensitivity of lung cancer cells. The silencing of the vacuolar ATPase gene ATP6V0D2, for example, sensitizes cells to paclitaxel, suggesting that the combination of paclitaxel and a lysosomal ATPase inhibitor could provide synergy. Indeed, the authors demonstrate a significant collaboration of the ATPase inhibitor RTA 203 with low-dose paclitaxel, thereby validating the tremendous ability of this synthetic-lethal screen to predict potent drug–drug interactions. [
] —PR McDonald, U Pittsburgh
Erythropoietin Therapy for Neurovascular Repair
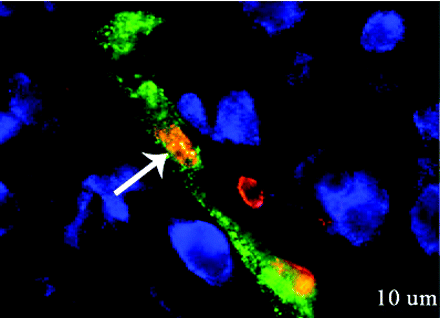
Ischemic stroke continues to be a leading cause of adult and neonatal death and morbidity. Erythropoietin (EPO) has been extensively tested as a potential therapeutic in animal models of stroke and in clinical but its effects on the brain are not fully understood. In the August issue of JPET, Keogh et al. report their investigation of a neonatal ischemic stroke model, in which they show that post-stroke treatment with recombinant human EPO (rhEPO) increases the expression of key neurovascular remodeling proteins, including angiopoietin-2, receptor tyrosine kinase Tie-1, and basic fibroblast growth factor. In addition to previous evidence that rhEPO reduces ischemia-induced neuronal cell death, Keogh et al. show that rhEPO treatment attenuates endothelial cell death and increases the proliferation of these cells. Interestingly, rhEPO increases the number of neurons in proximity to newly formed microvessels. These observations add insight into the mechanism of EPO-mediated effects and may lead to even broader applications of EPO therapy in neurological disorders. [
] —K Francis, Medical University of South Carolina.
- © American Society for Pharmacology and Experimental Theraputics 2007



