Targeted Delivery of Radioprotective Agents to Mitochondria
Abstract
Adverse effects of ionizing radiation are mediated through reactive oxygen and nitrogen species. Mitochondria are the principal source of these species in the cell and play an important role in irradiation-induced apoptosis. The use of free radical scavengers and nitric oxide synthase inhibitors has proven to protect normal tissues and, in some cases, to sensitize tumor tissues to radiation damage. Dual molecules that combine radical-scavenging and NOS-inhibitory functions may be particularly effective. Drugging strategies that target mitochondria can enhance the effectiveness of such agents, in comparison to systemic administration, and circumvent side effects.
Introduction
The development of agents that protect tissues against radiation damage (i.e., radioprotective agents) is currently the subject of intense research on at least two broad accounts. First, radiotherapy remains one of the most widely used treatments for cancer. Irradiation-induced DNA damage can halt tumor cell proliferation, but collateral radiation damage to surrounding tissues is always a concern. Accordingly, there is a need to develop drugs that will protect healthy cells while leaving malignant cells susceptible—and ideally, even sensitized—to radiation therapy. An additional impetus for research is the need to counteract occupational risks and terrorist threats of radiation exposure.
Damage to DNA, the primary target of radiation treatment, can occur directly, but most genetic damage is mediated by reactive oxygen and nitrogen species (ROS and RNS). Hence, scavengers of free radicals form the principal group of radioprotective agents. It has been hypothesized that irradiation produces bursts of ROS [e.g., superoxide (O2−) and hydroxyl (OH) radicals] by reacting with the aqueous environment of the cell. However, recent findings suggest that the principal origin of irradiation-induced free radicals is the mitochondria (1, 2). Accordingly, antagonists of nitric oxide synthase (NOS) are also of interest for potential radioprotective measures. A significant challenge to the use of radioprotective agents will be to deliver them through biological membranes and accumulate them at effective concentrations within mitochondrial domains where ROS and NOS are generated.
Irradiation-Induced Damage
The cellular response to irradiation is complex and includes genomic instability. Bystander effects have been frequently observed at low doses and show a non-linear response (3). The genotype and phenotype of the irradiated cell or animal as well as the nature of irradiation determine cellular response to irradiation (4). Cell types that are especially sensitive to irradiation in different organs are listed in Table 1⇓. Damage to DNA (e.g., double-strand breaks) triggers multiple signaling events, the variety of which extends to ataxia telangiectasia mutated (ATM) and Rad3-related protein; ERBB family and other tyrosine kinases (5, 6); protein kinase C; extracellular signal-regulated kinase 1/2 (ERK1/2) (7, 8); and increased production of ceramide (9).
Irradiation-Induced Organ Damage
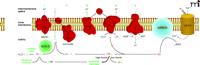
The initial cytotoxicity of irradiation, however, is believed to result from ROS and RNS generation (10). Ionizing radiation produces a burst of O2− in the cell within 10−13 seconds of radiation exposure. This free radical burst can damage DNA and proteins including mitochondrial respiratory complexes. Theoretical calculations suggest, however, that the initial ROS [i.e., O2− and its dismutation product hydrogen peroxide (H2O2)] formed during a clinical radiation dose [1–2 Gray (1 Gy = 1 J/kg = 100 rad)] may be etiologically insignificant. Indeed, much evidence suggests that ionizing radiation directly alters mitochondrial metabolism (11, 12) and transiently opens the unregulated mitochondrial permeability transition pore (MPTP). In particular, the opening of the MPTP causes an influx of Ca2+ into the mitochondrial matrix, thereby activating mitochondrial nitric oxide synthase (mtNOS). The resulting nitric oxide (NO) inhibits the respiratory chain (Figure 1⇓), thereby generating large amounts O2− that react with NO to form highly reactive peroxynitrite (ONO2−).
Schematic depicting the inner mitochondrial membrane and the five subunits of the mitochondrial electron transport chain: I, NADH dehydrogenase; II, succinate dehydrogenase; III, cytochrome c reductase; IV, cytochrome c oxidase; V, ATP synthase; CoQ, coenzyme Q; and Cytc, cytochrome c. Also included in the scheme are the putative locations of MnSOD, the mitochondrial permeability transition pore (MPTP), and mitochondrial NO synthase (mtNOS). In addition, the scheme shows our hypothesis for irradiation-induced activation of mtNOS, respiratory chain inhibition, and free radical generation. Adapted from (22).
Radioprotective Agents
Radioprotective agents must manifest one or more of the following properties: antioxidant avtivity, NOS inhibition, anti-inflammatory or immunomodulatory activity (Table 2⇓). Most of these existing drugs can neutralize ROS by a number of different mechanisms. As introduced above, the utility of radioprotective agents may rest on strategies for delivering them to the mitochondrion; features related to these strategies are presented in Table 3⇓.
Agents that Provide Protection against Radiation
Methods for Targeting Mitochondria
Thiol-Containing Compounds
Aminofostine is the most prominent compound of this class of drugs. The prodrug (WR2721) accumulates preferentially in the kidneys and salivary glands, where it is metabolized to the active WR1065 (13). It scavenges O2− as well as OH and lipoperoxyl (LOO) radicals (14). Aminofostine is protective in normal but not tumor cells, reportedly due to higher alkaline phosphatase activity, pH, and oxygenation in normal tissue. Aminofostine was approved by the Food and Drug Administration for patients undergoing radiotherapy for ovarian and head and neck cancers, but its intravenous administration can cause nausea, vomiting, and hypotension, which has limited its use. Studies are in progress to evaluate the effects of alternative routes of delivery (15).
Manganese SOD
Superoxide dismutases (SODs) are important components of the cellular antioxidant system, catalyzing the dismutation of O2− to O2 and H2O2 (16). There are three isoforms in mammalian cells that may vary with respect to the specific redox-active metal ion necessary for catalysis. The mitochondrial isoform of SOD (MnSOD or SOD2) is manganese-dependent and is called mitochondrial SOD. A specific N-terminal leader sequence directs the polypeptide to mitochondria and is then cleaved; MnSOD devoid of this leader sequence localizes to the cytosol and is not radioprotective (1, 2). Mitochondrial localization of MnSOD has been shown to be associated with decreased irradiation-induced apoptosis (17).
Nitroxides
Nitroxides are low molecular–weight compounds containing tertiary amine functional groups that are oxidized (R3N +-O−) to form relatively stable free radicals. They were initially used as probes for ESR spectroscopy and more recently were shown to have antioxidant activity. Many of them are water-soluble and act, as superoxide dismutase mimetics, to neutralize O2− and OH and organic hydroperoxides. Although nitroxides are cell membrane–permeable, they may not get into mitochondria. Using mass spectroscopy, we have shown that the nitroxide 4-amino-TEMPO is unable to penetrate mitochondrial membranes and requires targeting for effective antioxidant activity (18). Despite these findings, some nitroxides, including TEMPO, show radioprotective properties in vitro and in vivo in mice (when administered intraperitoneally [see (19)]). Among the positive characteristics of nitroxides is their selectivity for normal tissues; TEMPO is rapidly reduced to an inactive hydroxylamine in tumor hypoxic tissues (19).
Vitamins
There is experimental and clinical evidence that the level of vitamins is reduced in irradiated tissue. Supplemental diets including high doses of vitamins A, C, and E are protective in normal tissues during radiation therapy [reviewed in (14), (20)]. Moreover, when present in high doses throughout the treatment period, these vitamins have been shown to be radiosensitizing in some types of cancer cells. In this regard, it is thought that these vitamins each have a distinct mechanism of action, and thus a mixture of them is more effective (20). Although high doses of vitamins are necessary for beneficial effects, toxicity limits their use to particular organs. High dose–vitamin C can cause diarrhea; vitamin E is associated with defective blood clotting; and vitamin A is linked to skin bronzing.
Melatonin
Melatonin (N-acetyl-5-methoxytryptamine) is an endogenous compound synthesized by the pineal gland and released into the bloodstream (21). It is a potent antioxidant and can directly scavenge OH radicals as well as increase the activity of enzymes such as SOD and glutathione peroxidase (GPX). Melatonin decreases the activity of NOS and is radioprotective and nontoxic in vitro and in vivo [reviewed in (21)]. Melatonin is also reported to reduce tumor cell growth by inducing apoptosis or reducing invasiveness, underscoring its clinical potential.
NOS Inhibitors
Although they may not be generally considered first and foremost as radioprotective agents, NOS antagonists (e.g., l-NMMA, AMT) have been shown to prevent radiation cystitis more effectively than MnSOD transgene therapy (22). Their intravesical administration protects the umbrella cell layer of the urinary bladder from superficial ulcerations, thereby preventing changes in trans-epithelial resistance and permeability to water or urine. NOS antagonists may prevent both NO and O2− production, whereas the scavenging and dismutation of O2− produces H2O2, which must be neutralized by other antioxidant enzymes, such as GPX and catalase (Figure 1⇑). Since systemic administration of NOS inhibitors can cause hypertension, targeting them to mitochondria is therapeutically relevant.
Anti-inflammatory Agents
Inflammation can be a direct effect of irradiation as well as a secondary effect caused by oxidative stress. An acute inflammatory response involves activation of stress-sensitive kinases and transcription factors and the production of inflammatory cytokines (23). Because the chronic overexpression of cytokines and growth factors can result in fibrosis or necrosis, anti-inflammatory agents are used for radioprotection. Among these is palifermin, a recombinant human keratinocyte growth factor. This agent can stimulate cellular proliferation and differentiation in epithelial cells and enhance intrinsic glutathione peroxidase activity (13). Another radioprotective agent in this category is resveratrol (polyphenolic phytoalexin), which prosesses potent anti-oxidant, anti-inflammatory and anti-proliferative properties. Resveratrol has been shown to protect keratinocytes from UVB radiation–induced cell death by inhibiting caspases (24).
Immunomodulators
Hematopoietic cells have a high turnover rate and are thus extremely sensitive to irradiation. The immune system is affected and suppressed at relatively low doses of acute irradiation, reducing bone marrow cell production as well as inducing apoptosis in mature cells (25). Accordingly, compounds that regulate cytokine production can protect against irradiation damage and bring about repair through enhanced production of bone marrow cells, lymphocytes, platelets, and circulating granulocytes. Finally, radioprotective properties have been attributed to propolis (26), PR-1 (an extract of Podophylium hexandrum rhizomes) (27), and a number of other immunomodulatory agents.
Targeting Mitochondria
Proteins
To be properly recognized and imported into mitochondria, translated proteins require an N-terminal specific amino acid sequence (28, 29). The mitochondrial signal peptide can be experimentally linked to non-mitochondrial proteins to promote their uptake into the mitochondrial matrix; the mitochondrial protein import machinery includes the translocase of the outer membrane (TOM) complex and the translocase of the inner mitochondrial membrane (TIM) complexes (30). Proteins interacting with the TIM complexes are either integrated into the inner mitochondrial membrane or transported into the mitochondrial matrix and processed by the mitochondrial processing peptidase (MPP). Less frequently, proteins may be recognized by a C-terminal sequence consisting of twenty to thirty residues. Certain proteins encode the necessary recognition elements as parts of their primary sequence, in which case import occurs with minimal processing (31).
MnSOD-Plasmid/Adenovirus
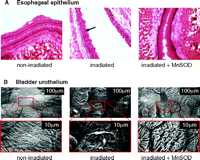
MnSOD gene therapy strategies for irradiation protection may be based on administration of a plasmidal MnSOD-encoding trans-gene carried within liposomes or adenoviruses. Intratracheal injections of either MnSOD-endocing plasmids or adenoviruses were shown to be protective against total lung irradiation in a mouse model (32–34); oral administration protected the mouse esophagus from irradiation-induced esophagitis (35) (Figure 2A⇓) and prevented oral cavity mucositis (36). Finally, intravesical instillation of MnSOD-encoding plasmid DNA twenty-four hours prior to irradiation protected bladders from radiation cystitis (Figure 2B⇓) (37).
Radiation damage and protection by MnSOD. A. Brightfield micrograph depicting changes that occurred in esophageal epithelium after irradiation (30 Gy). The non-irradiated esophagus showed normal intact epithelium (n=5). Alternatively, the arrow in the irradiated micrograph points to holes in esophageal epithelial cells which represent nuclei that have undergone apoptosis and are no longer present (n=5). Intraesophageal administration of MnSOD 24 hours prior to irradiation protected the epithelial cells from undergoing apoptosis (n=5). Adapted from (35). B. Scanning electron micrographs depicting changes that occurred in the bladder urothelium after irradiation. The bottom electron micrographs are enlargements of the areas enclosed by the rectangles in the top micrographs. The non-irradiated bladders showed normal intact urothelium (n=7). However, 48 hours after irradiation (35 Gy), the bladder urothelium showed areas of superficial ulcerations of the umbrella cells (n=6). In rat bladders transfected intravesically with the human MnSOD transgene 24 hours prior to irradiation, the urothelium showed only minimal ulcerations (n=5). Adapted from (22, 37).
Peptides
Several classes of cell-permeable antioxidant peptides that permeate into the inner mitochondria membrane have recently been used as targeting systems. One of the classes, known as Szeto-Schiller (SS) peptides [reviewed in (38, 39)], comprises tetrapeptides of alternating aromatic and basic residues. Although these peptides are cationic, they localize to the inner membrane rather than the matrix. Accordingly, their uptake is independent of the mitochondrial membrane potential and they concentrate rapidly (1000–5000-fold after two minutes) within mitochondria (40).
The antioxidant properties of SS peptides do not appear to be derived from the specific tetrapeptide sequence, although scavenging activity is highly dependent upon the inclusion of a dimethyltyrosine (DMT) residue. For example, DMT-d-Arg-Phe-Lys-NH2 scavenges as well as d-Arg-DMT-Lys-Phe-NH2; substitution of DMT with phenylalanine, however, abolishes scavenging activity (40). SS peptides have been shown to inhibit lipid peroxidation by scavenging OH radicals (40). They can reduce both spontaneous and Antimycin A–induced mitochondrial H2O2 production without uncoupling mitochondria (39). They also inhibit mitochondrial permeability transition and swelling (induced by calcium overload or inhibitors of the mitochondrial electron transport chain) (38). SS peptides are not cytotoxic and are effective at concentrations ranging from 0.1–100 nM (40, 41).
Gramicidin S Analogs
Another class of potential-independent peptides have been prepared through derivatization of the antibiotic gramicidin S (31). These gramicidin S analogs were used to target mitochondria with TEMPO (the SOD mimic discussed above) and the potent NOS inhibitor AMT (2-amino-6-methylthiazine) (18). Critical amide bonds in these compounds were replaced with (E)-alkene peptide isosteres which improved their bioavailability, possibly due to increased resistance against protease action (42–44), and the analogs could be structurally altered to modulate cell membrane passage relative to mitochondrial targeting. ESR spectroscopy and mass spectroscopy confirmed that the gramicidin S–based compounds successfully delivered and concentrated both TEMPO and AMT to mitochondria, whereas the presence of free drugs was not observed (18, 45). Harmful ONO2− production in irradiated cells was reduced or eliminated upon treatment with conjugated TEMPO and AMT, respectively (18). The ability of rats to survive lethal hemorrhagic shock was significantly increased by intravenous injection of the TEMPO-conjugated compound (46).
Cations
Another approach to target antioxidants to the mitochondria is to conjugate them to lipophilic cations. The inner mitochondrial membrane potential of 150–180 mV (negative inside) drives accumulation of cation-conjugated compounds into the mitochondrion. Triphenyl phosphonium (TPP), which was first applied by Murphy and coworkers (47), is the most commonly used cation. A wide range of antioxidants have been targeted to mitochondria by TPP, including tocopherol (48), ubiquinone (49), spin traps (50–52), the glutathione peroxidase mimetic ebselen (53, 54), and lipoic acid (55). Conjugation of ubiquinone to TPP (i.e., MitoQ) was found to be most effective as an antioxidant, accepting electrons from the mitochondrial respiratory chain (56). [Although toxicity owing to disruption of ATP synthesis has been regarded as a major limiting factor in therapeutic use (57), see (58).]
Radiosensitization of Tumor Cells
In studies of normal irradiated tissues (e.g., urinary bladder), the targeted delivery of NOS antagonists to mitochondria has proven more protective than O2− scavengers or MnSOD overexpression. Experiments with orthotopic tumors in mice were conducted to determine whether MnSOD overexpression results in radioprotection of tumor cells (59, 60). Interestingly, tumor radiosensitization was observed following intra-tracheal (17) and intravenous (61) administration of MnSOD-expressing plasmid liposomes, possibly reflecting a deficiency in coping with a MnSOD-mediated elevation of H2O2 levels (62). The precise role of H2O2 in tumor cells is not clear. MnSOD overexpression decreases the production of cytoprotective genes such as TNF-α, VEGF1, and IL-β, but increases GADD53, involved in DNA repair (17). Generation of H 2O2 may also beneficially modulate the EGF signaling pathway. In general, the toxicity of H2O2 in biological systems is considered to be due to its conversion to the highly reactive OH radical (63). The inner mitochondrial membrane contains many redox-active transition metals, such as iron and copper, that catalyze production of OH radicals (the Fenton reaction), which can lead to lipid peroxidation and loss of cytochrome c.
Dual-Action Compounds
Chabrier et al. synthesized one of the first dual-action compounds, Trolox, by coupling an antioxidant to a NOS inhibitor (thiophene amidine) via a piperidine linker. This compound has been shown to be neuroprotective in both rats and mice (64, 65) and to provide significant protection even when given four to eight hours after injury (66). Moreover, the therapeutic effect of the linked agents is greater than when the two agents are given unlinked (66). This may be due to the fact that NOS, when inhibited, can produce both NO and O2− (67, 68). Formed ONO2− may damage the NOS enzyme and worsen the chances for recovery from injury. Alternatively, the individual use of NOS inhibitors or O2− scavengers decreases the level of only one radical (NO or O2−) leaving the other free to act alone and elicit toxicity. The combined use of a linked NOS inhibitor and O2− scavenger has been shown to provide a synergistic effect in protecting tissues from ischemia in rats (69).
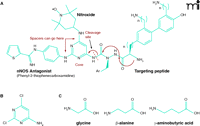
One approach to designing a dual-action compound (Figure 3A⇓) is to use 4-amino-2,6-dichloropyrimidine (Figure 3B⇓) as a core structure for ligation with radioprotective agents and targeting peptides or peptide isosteres. The halogen atoms of the heterocycle can be selectively displaced with amine nucleophiles (70, 71), and the amino function can serve as the attachment point for the peptide. Additional amino acid spacer groups (e.g., glycine, β-alanine, γ-aminobutyric acid; Figure 3C⇓) can be added between the radioprotective agents (nitroxide and NOS antagonist) as necessary to optimize therapeutic effects. The aminopyrimidine substructure is present in many pharmaceutical formulations and does not have adverse physiological or metabolic effects on its own (72) and is well-suited for the preparation of multiple-component agents. The example of a dual-action compound as given in Figure 3A⇓ incorporates an NOS antagonist and a nitroxide derivative. Other compounds can employ an SOD mimetic in place of the nitroxide (Figure 4⇓).
Scheme for dual-action drug ligation using a pyrimidine core and amino acid spacer groups. A. As a core structure to ligate mtNOS inhibitors, ROS scavengers (e.g., TEMPO derivatives) and targeting peptide, one can choose a commercially available compound such as 4-amino-2,6-dichloropyrimidine B. The halogen atoms on this heterocycle can be selectively displaced with amine nucleophiles, and the amino function can serve as the attachment point for the targeting peptide. C. Additional bifunctional amino acid spacer groups (e.g., glycine, β-alanine or γ-aminobutyric acid) can be readily introduced (unpublished data).
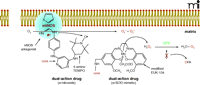
The right combination. Scheme for the mechanism of a dual-action drug with an NOS antagonist bound to NOS, resulting in electrons being transferred to O2 instead of the normal substrate, L-arginine. A pyrimidine core connects the NOS antagonist (e.g., Phenyl-2-thiopenecaraboxaminide) to a nitroxide (e.g., 4-amino-TEMPO) which can scavenge electrons and prevent O2− formation. An SOD chemically modified mimetic (e.g., EUK-134) may be used in place of the nitroxide derivative in the dual-action drug. However, because SOD mimetics scavenge and convert O2− to H2O2, the highly reactive OH radical may be formed if glutathione peroxidase (GPX) activity decreases. Therefore, a SOD mimetic that exhibits intrinsic GPX/catalase-like activity should be employed. Adapted from (18).
Conclusions
The development of radioprotective agents to spare healthy tissue during focal radiation therapy and to protect against occupational and terrorist-initiated radiation exposure is the subject of intense research. Ionizing radiation generates free radicals that damage DNA and kill cells, particularly those that are rapidly dividing, such as in tumors. Although it was initially believed that free radicals were generated mainly in the cytosol, it is becoming clear that the principal site of their formation is within the mitochondria. Accordingly, targeting and concentrating free radical scavengers to mitochondria, using various delivery strategies, offers the prospect of systemic administration of selective agents at lower concentrations and with fewer adverse side effects. Recent findings also suggest that an upstream site of free radical generation is mtNOS, whose inhibition can circumvent the need for dismutation and the formation of H2O2. However, in tumor cells where peroxidase levels may be low, the use of MnSOD may result in excess H2O2, which beneficially sensitizes these cells to ionizing radiation. Thus, an NOS antagonist or free radical scavenger may be beneficial in protecting healthy tissue, whereas MnSOD may be advantageous in sensitizing cancerous tissue. Finally, the inhibition of NOS can result in brief periods of simultaneous NO and O2− production, resulting in the formation of ONO2−, which may damage the enzyme as well as the surrounding tissues. This fact may account for beneficial effects seen when using dual-action compounds containing an NOS antagonist and a free radical scavenger, relative to the use of the two active moieties unattached in a cocktail.
Acknowledgments
Our research was supported by grants from the American Cancer Society (RSC-03-077-CNE), the National Institutes of Health (DK 071085) and the 2008 ASPET-Astellas Award for Translational Pharmacology (to AJK).
- © American Society for Pharmacology and Experimental Theraputics 2008
References

Irina Zabbarova, PhD, is Research Fellow at the University of Pittsburgh School of Medicine. Her research has focused on the generation of reactive oxygen species and reactive nitrogen species in biological systems. Her recent work has focused in part on the radioprotective effects of radical scavengers and inhibitors of nitric oxide synthase.

Anthony John Kanai, PhD, is Assistant Professor of Pharmacology at the University of Pittsburgh School of Medicine. He is the recipient of the 2008 ASPET-Astellas Award in Translational Pharmacology. His research pursues the therapeutic potential for treatment and prevention of radiation damage and malignancies.