Therapeutic Windows
Fluoxetine (Prozac®)
But for the intercession of key scientists and several intervening events, fluoxetine might have remained undeveloped by Eli Lilly and Company. As a runaway blockbuster, it greatly widened the dimensions of public pharmaceutical discourse, highlighting and perhaps even mitigating the social stigma of mental disease; reinvigorating experimental psychopharmacology; and stirring public health debates in psychiatry.

Discovery
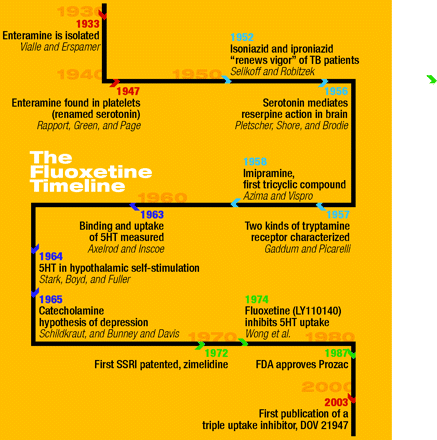
By the mid 1950s, the concept that emotions and behavior could be linked to chemical systems within the human brain was new and exciting. Pills for the Mind was the title of a 1955 piece in Time magazine, which described the dramatic transformation of patients at St Elizabeths Hospital, in Washington DC, whose psychoses largely succumbed to treatment with chlorpromazine or reserpine (1). Reserpine was really something of a re-discovered drug, known as an antihypertensive agent with the untoward effect of causing major depression in about twenty percent of patients. In a classic set of experiments from “Steve” Brodie’s Laboratory of Chemical Pharmacology at the NIH, the depressant-like effects of reserpine were solidly linked to brain serotonin (2, 3). Brodie also established that LSD functioned as an antagonist of serotonin-mediated reserpine action. A decade later, Ray Fuller, a pharmacologist at Eli Lilly and Company, took note of Brodie’s work and pursued the role of serotonin in the central reward system of rodents and dogs (4). As the tricyclic antidepressants came into clinical use in the 1960s, the inhibited reuptake of norepinephrine and serotonin in nerve terminals was posited as a therapeutic mechanism. In the late 1960s, pharmacologist Arvid Carlsson described a compound (i.e., zimelidine) that acted as a selective serotonin reuptake inhibitor (SSRI) [see (5)]. But it was through the imagination, research, and management efforts of Ray Fuller and colleagues at Eli Lilly that fluoxetine exploded onto the drug market, under the trade name Prozac, in the late 1980s.

Indicated uses
-
Major depressive disorder
-
Maintenance of euthymia
-
Obsessive compulsive disorder
-
Bulimia nervosa
-
Panic disorder
-
Premenstrual dysphoric disorder
Reminiscences on Prozac
The development and marketing of Prozac, along with the impact of Prozac’s unprecedented success, have been treated, either favorably or scathingly, in a number of books published since the 1980s. Many of these come with catchy titles, like Listening to Prozac, or Let Them Eat Prozac. Any attempt to summarize the merit and gravity of these various treatises is beyond the scope of Therapeutic Windows. Let it suffice here to quote Elizabeth Wurtzel, admittedly out of context, from her cleverly entitled memoir (see book cover, right): “It seemed like this was one big Prozac nation, one big mess of malaise.”

On the other hand, the scientific development of fluoxetine has been rather neatly documented in several reviews and less formal personal histories. Ray W. Fuller is generally credited as a leading member of the scientific team, along with David Wong and Bryan Molloy, who drove the development of Prozac at Eli Lilly. One of the group’s own reminiscences attests to the thoughtfulness and reserved optimism of their research (6): “An uptake system on the serotonin neuronal membrane apparently functions to inactivate serotonin that has been released into the synaptic cleft.…Through the use of fluoxetine and other serotonin uptake inhibitors, the role of serotonin neurons in various brain functions behavior, sleep, regulation of pituitary hormone release, thermoregulation, pain responsiveness, and so on can be studied.”
Indeed, if one goes back to the pharmacology literature, including contributions from Gaddum, Brodie, Axelrod, Fuller, and many more, the implication of serotonin and other brain chemicals in mammalian behavior and human emotion is anything but “messy.” It remains a remarkable pharmacological achievement still exciting to think of today that reasoned experiments could have resulted in a drug that targets the reuptake of an essential brain neurotransmitter without commensurately interfering with receptor function.
On the market
Fluoxetine earned FDA approval in 1987, thirteen years after its first appearance in the scientific literature as LY110140. “Lilly’s market assessment indicated that antidepressants were less than a $500 million market, about $200 million of which was domestic.” Lilly’s own people expected $70–$100 million in sales for the first year. “First year sales were $130 million and within 24 months, sales exceeded the total sales for all [other] antidepressants. After two years on the market, the drug generated about 25 percent of Lilly’s sales (upwards of 35 percent worldwide), a situation that lasted for years and drove the growth of the company throughout the 1990s. Yearly sales eventually reached $3 billion worldwide. Prozac quickly became the biggest-selling drug in history and redefined what was meant by the term blockbuster drug (7).”
Ins and outs
-
a single oral dose (40 mg) yields peak plasma concentrations of 15 to 55 ng/mL
-
multiple doses (40 mg/day for 30 days) yield a peak concentration of 91 302 ng/mL and 72 258 ng/mL for fluoxetine and norfluoxetine, respectively
-
in vitro 94.5% of fluoxetine is bound by serum proteins
-
the R and S enantiomers have equivalent pharmacological activity
-
fluoxetine is metabolized primarily through demethylation by cytochromes P450 CYP2D6 to norfluoxetine, the major metabolite
-
S-norfluoxetine is nearly as potent as the racemic parent compounds but R-norfluoxetine is much less potent at inhibiting 5HT uptake
-
fluoxetine is excreted in human milk
-
fluoxetine and its metabolites are excreted 80% in urine and 15% in feces
Action and adversity
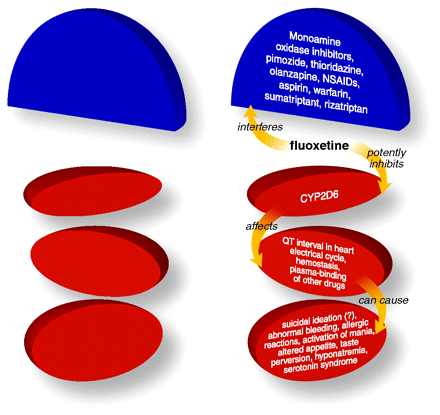
Fluoxetine blocks the reuptake of serotonin from the synaptic cleft by serotonin transporters without binding post-synaptic or presynaptic serotonin receptors. Fluoxetine is metabolized by CYP2D6 but also acts as a potent inihibtor of this P450 isozyme.
Footnotes
- © American Society for Pharmacology and Experimental Theraputics 2009