Emerging concepts from the recent literature
Hydrogen Sulfide as Hypertension Regulator
Hypertension is one of the risk factors for stroke, heart disease, and heart failure—serious causes of morbidity and mortality in the developing world. Hydrogen sulfide (H2S) has previously been shown to promote vascular relaxation and thereby decrease blood pressure; however, the origin of endogeneous H2S and the mechanism of action have remained unclear. In the June issue of JPET, Xia et al. identify two enzymes, cystathionine β-synthetase (CBS) and cystathionine γ-lyase (CGL), as producers of H2S in the kidney. Using a rat model of renal function, the authors demonstrate H2S production by these enzymes and present evidence that H2S may act through inhibition of a Na+/K+/2Cl− cotransporter and a Na+/K+/ATPase. This report provides insight into the underlying mechanism of H2S control of blood pressure regulation and opens the way for further understanding of the biological basis of hypertension. [Xia, M., Chen, L., Muh, R.W., Li, P. L., and Li, N. Production and actions of hydrogen sulfide, a novel gaseous bioactive substance, in the kidneys. J. Pharmacol. Exp. Therap. 329, 1056–1062 (2009).] —DD Dudgeon, University of Pittsburgh
Stauprimide: A Small Molecule Directs Stem Cells to Differentiate
Successful application of stem cell technology for developing laboratory research models and for therapeutic purposes requires precise control of stem cell differentiation in vitro. Recently, several groups have screened small-molecule libraries for novel chemical compounds that can direct differentiation of embryonic stem cells (ESCs) into specific cell types. In a screen of 20,000 compounds from a kinase-orientated library, Zhu et al. have identified a small molecule, termed stauprimide, that significantly enhances the efficiency of directed human and mouse ESC differentiation, in response to lineage specification cues, into definitive endoderm as well as neural or mesodermal lineages. Intriguingly, stauprimide interacts with NME2, a transcription factor that regulates expression of c-Myc, a key regulator in the maintenance of ESC self-renewal. Knockdown of NME2, like stauprimide treatment, specifically decreases c-Myc expression and may thereby promote the differentiation of ESCs. The authors further demonstrate that stauprimide causes the downregulation c-Myc by inhibiting the nuclear localization of NME2. [Zhu, S., Wurdak, H., Wang, J., Lyssiotis, C.A., Peters, E.C., Cho, C.Y., Wu, X., and Schultz, P.G. A small molecule primes embryonic stem cells for differentiation. Cell Stem Cell 4, 416–426 (2009).] —F Zhang, U Pittsburgh
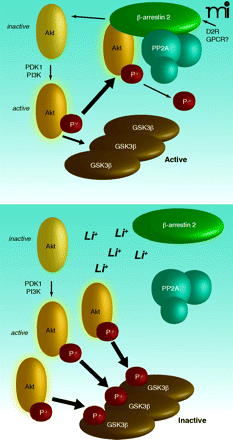
Paradoxical Akt Activation by Chemical Akt Inhibitors Unraveled
The protein kinase Akt is a central mediator of cell survival but is often aberrantly activated in tumors. Chemical inhibitors of Akt have thus inspired great interest. Paradoxically, however, pharmacological blockade of Akt with competitive inhibitors of the ATP-binding site triggers two activating phosphorylation events on Akt. Is there an intrinsic change to Akt upon inhibitor binding that promotes its phosphorylation? Or is another mechanism, such as relief of an upstream negative (ATP-binding) regulator, involved? To address this question, Okuzumi et al. have used a clever chemical genetic approach to examine a form of Akt uniquely capable of binding bulky ATP analogs. The authors found that the ATP analog–bound Akt variant is recruited to the cell membrane, where it is rapidly phosphorylated by upstream activators. This elegant study underscores a potential flaw in the targeting of Akt for cancer therapy. Inhibitor binding to the APT site of Akt may, within the complex in vivo cellular context, act to prime, rather than hinder, cancer-associated signaling pathways. [Okuzumi, T., Fiedler, D., Zhang, C., Gray, D.C, Aizenstein, B., Hoffman, R., and Shokat, K.M. Inhibitor hijacking of Akt activation. Nat. Chem. Biol. 5, 484–493 (2009).] —R Tomko, Yale U
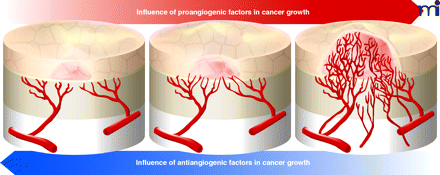
Thalidomide: Developing Insights into Developmental Defects
Recently, the Figg and Vargesson groups presented evidence in PNAS that the teratogenicity associated with thalidomide exposure is dependent on the maturity of the host’s vasculature. Using a previously identified fluorinated analog of a thalidomide metabolite (CPS49), which possesses antiangiogenic properties, the authors use a chick embryo model of development to illustrate that limb defects are clearly associated with a reduction in vessel density in newly forming appendages. The authors further validate this hypothesis by showing that CPS49 possesses similar effects in primary cell cultures and zebrafish embryos. Interestingly, due to the short half-life of CPS49, the temporary inhibition of vessel growth seen in mature, stable tissues was followed by a period of recovery, whereas immature tissues were irreversibly lost. This work is not only important because it adds much needed developmental stage-specific data to the thalidomide literature, but also because it provides further support that specific analogs of thalidomide possess angiostatic properties and therapeutic potential. [Therapontos, C., Erskine, L., Gardner, E.R., Figg, W.D., and Vargesson, N. Thalidomide induces limb defects by preventing angiogenic outgrowth during early limb formation. Proc. Natl. Acad. Sci. USA 106, 8573–8578 (2009).] —EE Gardner, U Pittsburgh
- Copyright © 2009



