Regenerative Pharmacology for Diabetes Mellitus
Regenerative medicine utilizes cutting-edge technologies to repair and replace damaged cells, tissues, and organs for functional restoration. This broadly interdisciplinary field draws from cell and molecular biology, genetics, immunology, surgical sciences, physiology, biomedical and tissue engineering, chemical and material sciences, and nanotechnology. Certainly, pharmacology serves as an integral component of regenerative medicine, a concept first elucidated in depth in this journal (1). We operationally define regenerative pharmacology as the application of pharmacological sciences to accelerate, optimize, or characterize the development, maturation, and function of bioengineered and regenerating tissues, either in vitro or in vivo. Diabetes mellitus, a condition defined by elevated levels of glucose in the blood, represents a compelling target for regenerative medicine, through a variety of complementary strategies (Figure 1). A recent report from the laboratory of Peter Schultz of small molecules capable of stimulating the proliferation of insulin-producing pancreatic β cells highlights the potential of regenerative pharmacology to develop novel treatments for this important disease (2).
In Type 1, or juvenile diabetes (T1DM), autoimmune-dependent destruction of β cells in the islets of Langerhans of the pancreas severely decreases the production of insulin, the hormone that signals cells throughout the body to take up and metabolize glucose. In Type 2 diabetes (T2DM), impaired responsiveness (“resistance”) of fat and muscle cells to insulin is coupled with deficiencies both in the net mass of pancreatic β cells and their capacity for regulated insulin secretion. Although the injection of insulin is life saving in T1DM, and, together with other drugs, can help to control T2DM, current treatments allow few affected individuals to achieve normal glucose homeostasis. The imperfect metabolic control has devastating long-term consequences, especially as hyperglycemia causes widespread neural and vascular damage leading to secondary diseases and end-organ complications. In both forms of diabetes, the restoration of sufficient numbers of functional β cells would represent an important step towards definitive therapy.
Transplantation of human pancreatic islets from cadaveric donors has become a clinical reality for patients with severe T1DM who do not regulate glucose well with insulin therapy (3). Despite significant advances, islet transplantation still shows a relatively limited duration of efficacy. Whereas about two-thirds of islet transplant recipients show significant long-lasting benefits, such as reduced frequency of severe hypoglycemic episodes, only about 13% remain insulin-independent beyond five years (4). Moreover, the scarcity of donor organs and the intrinsic inefficiency in obtaining critical β-cell mass (i.e., many recipients require islets from two or more pancreata) imply that new ways to augment the supply of β cells will be essential in order for regenerative medicine to help the vast majority of individuals with diabetes (Figure 1). One possible solution is to generate β cells from embryonic or adult stem cells (5). Another potential solution, the goal of Wang et al., is to amplify β cells, either from a donor or from a patient’s own residual β-cell mass (2).
It is often assumed that mature, differentiated cells have little capacity for mitotic division and that their ongoing production depends entirely on progenitor and stem-cell populations. This model appears to hold in rapidly renewing tissues such as blood or the lining of the gut. However, lineage-tracing experiments show that differentiated β cells, which normally turn over slowly, are capable of significant proliferation (6, 7). Therefore, both mature cells and progenitors potentially could contribute to expansion of the β-cell mass in regeneration (8).
From a clinical perspective, the ability to increase substantially the number of β cells in culture prior to islet transplantation should make the therapy more effective and increase the number of patients who could benefit. Furthermore, the majority of individuals with T1D, and virtually all with T2D, retain substantial numbers of β cells (9). Conceivably, a drug could be developed to increase the mass of functional β cells in the pancreata of patients in vivo without the need for transplantation.
As an initial step towards a regenerative pharmacology strategy for diabetes, Wang et al. carried out high-throughput screening (HTS) to identify small molecules that stimulate β-cell replication. They describe a diverse set of “hits” that reveal potential targets for further drug discovery and development (2). Because human β cells are in scarce supply, the team screened against an engineered mouse β-cell line conditionally immortalized by a viral oncoprotein (SV40 T-antigen, T-Ag). The cells, originally isolated by Efrat and colleagues (10), grow extensively in culture when T-Ag is expressed, so they can be obtained in large quantities. However, when T-Ag is shut off (e.g., by withdrawal of an inducer, in this case, tetracycline), the cells undergo growth arrest and further mature to closely resemble adult β cells in their synthesis and glucose-regulated secretion of insulin and their ability to reverse diabetes (10). Furthermore, the growth-arrested cells can reenter the cell cycle when exposed to mitogenic growth factors known to act on normal pancreatic islet cells (11).
Wang et al. report that from a heterocycle library comprising ~850,000 compounds, about eighty (~0.01%) reproducibly induced proliferation of the test mouse β-cell line and also of normal rat β-cells (a secondary screen). These molecules represented ten distinct scaffolds and showed activity in the low micromolar range. The investigators identified probable targets for several classes of the hit compounds and present data on two: 1) agonists of the developmentally important Wnt/β-catenin signaling pathway; and 2) agonists of L-type calcium channels (LTCCs) (2). In this regard, it was already known that purified Wnt3a protein stimulates β-cell proliferation via a well-defined signaling pathway (12). The prototype small-molecule Wnt agonist, a thiophene pyrimidine derivative, designated compound 1a, stimulated reporter-gene expression in a Wnt-sensitive cell line with an intact signaling pathway, but not in a mutant line defective for the mobilization of β-catenin to the cell nucleus (2). In addition, Sulindac, a Wnt-signaling antagonist, blocked the activity of compound 1a. Finally, the authors found that the probable mechanism of Wnt pathway activation by compound 1a is inhibition of the serine–threonine protein kinase GSK3-β, a widely expressed negative regulator of Wnt signaling—thus, an inhibitor of this kinase serves as an agonist for the overall signaling pathway.
Molecules in the second class of HTS hits, represented by compound 2a, are dihydropyridine derivatives, a family that includes agonists and antagonists of LTCCs. A known LTCC agonist, Bay K 8644, also stimulated growth of the test cells. Conversely, the antagonist nimodipine interfered with growth stimulation by compound 2a; nimodipine alone induced death of the mouse β cells. As would be expected, exposure to the LTCC agonists caused a strong, transient increase in intracellular calcium. The proliferation of the test cells required the continuous presence of the LTCC agonist and was not accompanied by a decrease in insulin expression; dedifferentiation of the cells to a less mature, progenitor-like state did not appear to occur.
The identification of compounds with familiar pharmacological activity that are able to drive expansion of mature β cells validates the phenotype-based HTS strategy. Discovery of the molecular targets for additional classes of compounds can be anticipated through the use of a number of powerful tools, including affinity chromatography, sensitive proteomic analysis, and expression cloning, coupled with a wealth of genomic information (13, 14). This would logically be followed by the synthesis of more potent lead compounds that can be tested stringently for safety and efficacy in animal models of diabetes. The hurdles in the progression from the initial identification of HTS hits to the approval of a new chemical entity for direct human therapy are well appreciated. However, successful development of a molecule that simply promotes ex vivo proliferation of β cells, in order to increase substantially the total mass of cells available to transplant into patients with diabetes, seems a worthwhile and more immediately achievable translational goal.
The potential to develop a drug that would expand the remaining endogenous β-cell mass in patients with diabetes is more difficult to assess. Understanding of the relationship between normal organogenesis and regeneration remains relatively primitive (15). Developmental studies have revealed extrinsic signals (e.g., growth factors and extracellular matrix components) and cell-intrinsic regulators (e.g., transcription factors) that are essential for the formation of pancreatic β cells (16). However, the interactions of these elements are complex, and most of them are utilized in multiple tissues. Furthermore, many of the key factors may not be readily “druggable.” It appears a tall order to select an optimal target for a small molecule to promote the selective, controlled expansion of a single cell type to recapitulate a specific developmental pathway for pancreatic islet regeneration. Nonetheless, well-designed screening and lead development may identify compounds sufficiently selective to stand as legitimate drug candidates.
Advances in stem-cell biology further brighten the promise of regenerative pharmacology for diabetes. By mimicking sequential steps in normal pancreatic development, it has been possible to direct the differentiation of human embryonic stem cells to insulin-producing β-like cells, albeit at relatively low efficiency (17–20). Very recently, Douglas Melton and colleagues have identified small molecules from compound libraries that strongly promote two of the critical steps in the pathway: the commitment of pluripotent stem cells to the endodermal germ layer, and then further commitment to the pancreatic lineage (21, 22). These data, taken together with the results of Wang et al., may portend new avenues for the production of functional cells for transplantation, including cells genetically matched to the patient (23). In conclusion, there is strong reason to predict that the influence of pharmacology in regenerative medicine will continue to grow, especially as remarkable new biological discoveries motivate and enable screens for compounds with previously unanticipated activities. The advances in regenerative pharmacology promise truly innovative therapies for diabetes and many other disorders.
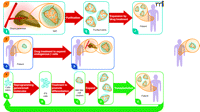
Three regenerative pharmacology strategies for diabetes treatment. Strategy 1. From donated pancreas Islets of Langerhans (A) are purified (B) and incubated with a small molecule drug that stimulates proliferation of β cells (colored orange; non-β cells in blue). Treated islets with enhanced β-cell mass (C) are transplanted into a recipient with diabetes. Patient requires immunosuppressive drugs to avoid transplant rejection. Strategy 2. Patient with diabetes has fewer β cells in shrunken islets (A). Treatment with a drug that stimulates proliferation of these endogenous cells expands the β-cell mass without need for islet transplantation (B). Strategy 3. Cells from a patient with diabetes are obtained from a minimally invasive biopsy—e.g., skin, hair, or peripheral blood—and grown briefly in culture (A). Induced pluripotent stem (iPS) cells are obtained by reprogramming the cells using a small set of pluripotency genes and/or small molecules (B). The iPS cells are differentiated in vitro to pancreatic islet-like clusters (ILCs) containing β cells using known biological factors and/or small molecules (C). The β cells in these structures may be further expanded [(D)], as in Strategy 1, and then transplanted back into the patient (E). The ILCs are transplanted into the patient. Immunosuppressive therapy is not required to prevent rejection. The strategy also could be used beginning with embryonic stem (ES) cells from an unrelated individual. In this case patients would require immunosuppressive drugs. None of these strategies addresses underlying causes of diabetes, such as autoimmunity (T1D) or insulin resistance (T2D), which must be addressed by additional treatments. However, increased functional β-cell mass would significantly improve regulation of blood sugar. Broad red arrows indicate points of possible pharmacological intervention.
- Copyright © 2009
References

George J. Christ, PhD, is Professor at the Wake Forest Institute for Regenerative Medicine. His broad interests include muscle physiology and pharmacology, intercellular communication, and the role of smooth muscle in the function and dysfunction of visceral and vascular tissues. He has published numerous studies on the impact of diabetes mellitus on end organ function, and has more recently turned his attention to applying the principles of pharmacology to regenerative medicine technologies. Address correspondence to GJC. E-mail gchrist{at}wfubmc.edu; fax 336-713-7290.

Mark E. Furth, PhD, is Technology Development Officer at the Wake Forest Institute for Regenerative Medicine. He received his doctoral degree in Molecular Biology from the University of Wisconsin-Madison for work on control elements for DNA replication, and did postdoctoral research in stem cell biology and oncogenes at the MRC Laboratory of Molecular Biology, Cambridge, UK, and the National Cancer Institute, Bethesda, MD. He headed the Laboratory of Molecular Oncogenesis at the Memorial Sloan-Kettering Cancer Center, and then spent eighteen years in executive research positions in the biotechnology and pharmaceutical industries. His current research focuses on medical applications of stem cells.