|
 
 |
| ORIGINAL ARTICLE |
|
| Year : 2014 | Volume
: 2
| Issue : 2 | Page : 54-58 |
|
Comparative histomorphological study of the stomach of Rattus norvergicus, Agama agama, and Bufo marinus
Joseph A Nwafor1, Ferdinand A OM' Niabohs2
1 Department of Anatomy, Federal University, Ndufu Alike Ikwo, Ebonyi State, Nigeria
2 Department of Anatomy, University of Benin, Benin, Edo State, Nigeria
| Date of Web Publication | 23-Mar-2015 |
Correspondence Address:
Joseph A Nwafor
Department of Anatomy, Federal University, Ndufu Alike Ikwo, Ebonyi State
Nigeria
 Source of Support: None, Conflict of Interest: None  | Check |
DOI: 10.4103/2315-7992.153817

Introduction: The histological view of the stomach presents an adaptation favoring the diets of each particular animal. The histoarchitectural organization, including the distribution of connective tissue fibers, provides a useful interpretation of the adaptative mechanisms adopted by the guts of different animals in coping with their diets. The rat is a mammal; the lizard, a reptile; and the toad, an amphibian. Their modes of diet are different and, as such, the organs involved in their diets show variation. Materials and Methods: We aimed to make a comparative study of the stomachs of the three types of animals in relation to their diet. Five adult Wistar rats, five adult agama lizards, and five adult cane toads of the same sex were used for this investigation. Result: The results revealed a sharp contrast in the histology of the stomachs among these vertebrates. A cursory look into the morphology of the stomach with regard to its shape and size also revealed significant differences. Conclusion: All of these observations on the histomorphologic pattern of the stomachs of these vertebrates suggest an adaptation in coping with their respective diets. Keywords: Cane toad, comparative biology, diet, lizard, morphology, rat, stomach
How to cite this article:
Nwafor JA, OM' Niabohs FA. Comparative histomorphological study of the stomach of Rattus norvergicus, Agama agama, and Bufo marinus. Ann Bioanthropol 2014;2:54-8 |
| Introduction | |  |
The morphology of the stomach is characterized by the thickened muscle arising from the mesoderm and unique glands derived from the endoderm. These muscles allow for elastic distension of the stomach and its peristaltic movements, which are required for the mechanical mixing of food with the glandular secretory juices for the purpose of digestion.
The histological view of the stomach presents an adaptation favoring the diet of each particular animal. [1] The histoarchitectural organization, including the distribution of connective tissue fibers, provides a useful interpretation of the adaptive mechanism adopted by the gut of different animals in coping with their diets. [2]
A few observations have been made that the reptilian stomach produces hydrochloric acid HCL. [3],[4],[5]
An extensive histochemical investigation on glycoconjugates in the fundic part of the stomach of Rana ridibunda showed a folded mucosa and luminal surface, and gastric pits lined by a simple columnar mucosa secreting superficial and foveolar cells. The luminal epithelium formed the fundic gastric glands that emptied into the base of the gastric pits and penetrated deeply into the lamina propria. The fundic glands were mostly of a simple tubular type, composed of foamy mucous neck cells and intensely eosinophilic cells. [6]
By defining the expression patterns of several genes within the developing guts, Smith et al., [7] compared the histoachitecture of the stomach and gastrointestinal tract in Xenopus laevis, Galhtsgalhis, and Mus musculus. The anterior portion of the stomach was reported to have a similar glandular histology as well as a common embryonic expression of the secreted factors WNT5A and BMP4. Likewise, within the amniote lineages, the posterior nonglandular stomach and pyloric sphincter regions were also comparable in both histological and molecular phenotypes. The posterior stomach expresses SIX2, BMPR1B, and BARX1, whereas the pyloric sphincter expresses NKX2.5. It was concluded that the global patterning of the gut is remarkably similar among the different vertebrate lineages.
Ofusori and Caxton-Martins [8] verified the comparative histomorphometric adaptations in the stomachs of rat, bat, and pangolin in relation to diet. It was reported that the cellular diameter/density of parietal and zymogenic cells are significantly different in the three mammals with the exception of the diameter of the zymogenic cells in the pangolin, which was not statistically significant when compared with that of the rat.
Studies have revealed that all these observations were reflections of the different patterns stomachs have adopted to cope with the animals' respective diets.
In the present study, we aimed at investigating the phylogenetic relatedness of apparently homologous organs of the stomachs of adult Wistar rat, agama lizard, and cane toad, as data on this subject appear to be lacking.
| Materials and methods | |  |
Materials
Chloroform, cotton wool, weighing balance, distilled water, ethanol, xylene, specimen bottles, sodium chloride, formaldehyde, measuring glass cylinder, rotary microtome, water bath, oven, disposable gloves, dissecting kit and board, slides and cover slips, biocular microscope with digital camera, and cages were used for this study.
Animals
The following animals were used for this study:
Five adult male cane toads (Bufo marinus) with an average weight of 45-60 g.
Five adult male agama lizards (Agama agama) with an average weight of 50-65 g.
Five adult male Wistar rats (Rattus norvegicus) with an average weight of 150-165 g.
Both the cane toads and the agama lizards were sourced within and around the University of Benin campus, Benin City, Nigeria; they were made available 96 h before sacrifice The rats were obtained from the animal house of the Department of Anatomy, University of Benin; they were allowed to acclimatize for a minimum period of 2 weeks.
All the animals were then kept in cages and maintained in the animal holding area of the Department of Anatomy, University of Benin, Benin City. During the period of experimental study, the animals were fed ad libitum with feeds that corresponded to their typical diets and with water ad libitum. The toads and the lizards were fed with insects and the Wistar rats were fed commercial grower's mash obtained from Bendel Feeds and Flour Mills, Ltd. in Benin City.
Method of sacrifice and tissue collection
The experimental animals were sacrificed by application of anesthesia with chloroform inhalation. The stomach part of the alimentary tract was harvested from each experimental animal following midline abdominal incision using a sharp scarpel and quickly fixed in 10% formal saline for routine histological analysis.
Tissue preparation for light microscopy
Tissue preparation was done for histological analysis. Tissues already fixed in 10% formol saline, after whole body perfusions were transferred to a graded series of ethanol. On day 1, they were placed in 70% alcohol for 7 h, then transferred to 90% alcohol and left in the latter overnight. On day 2, the tissues were passed through three changes of absolute alcohol for 1 h each and then cleared in xylene. Once cleared, the tissues were infiltrated in molten paraffin wax in the oven at 58°C.
Three changes of molten paraffin wax at 1-h interval were made, after which the tissues were embedded in wax and blocked out. Serial sections 5 μm thick were obtained from a solid block of tissue, fixed on clean slides coated with Mayer's egg albumin to cement the sections to the slides properly, and stained with hematoxylin and eosin stains; after this, they were passed through a mixture of equal concentration of xylene and alcohol. Following clearance in xylene, the sections were oven-dried at a temperature 35-40°C (Sheehan and Hrapchak, 1987). [9]
| Results and discussion | |  |
This study revealed conspicuous differences in the histology and morphology of stomachs among different vertebrates [Figure 1], [Figure 2], [Figure 3], [Figure 4], [Figure 5], [Figure 6], [Figure 7] and [Figure 8]. The mucosa of the stomach is specialized for secretory functions. There were variations in the muscularis mucosae of the stomach, which functions to produce local movement and folding of the mucosa. In mammals, the submucosa is that of a typical loose collagenous supporting tissue. It supports the mucosa layer and itself contains large blood vessels, lymphatics, and nerves.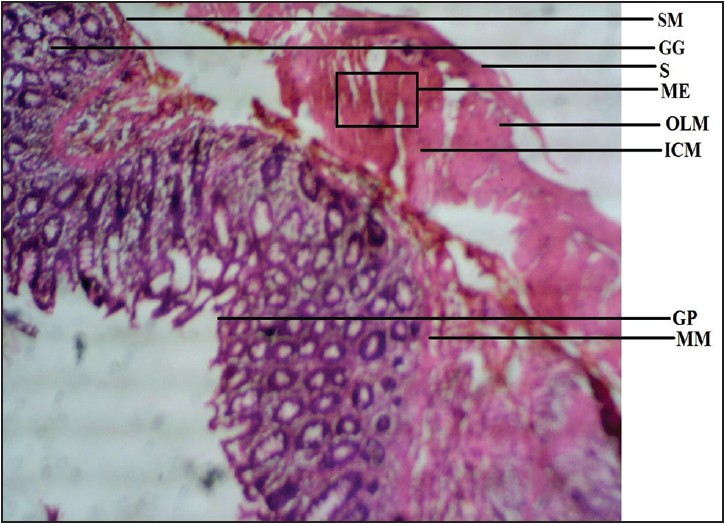 | Figure 1: Photomicrograph of rat stomach showing submucosa (SM), gastric gland (GG), serosa (S), muscularis externa (ME), outer longitudinal muscle (OLM), inner circular muscle (ICM), gastric pit (GP), and muscularis mucosae (MM). Stained with H&E (40×)
Click here to view |
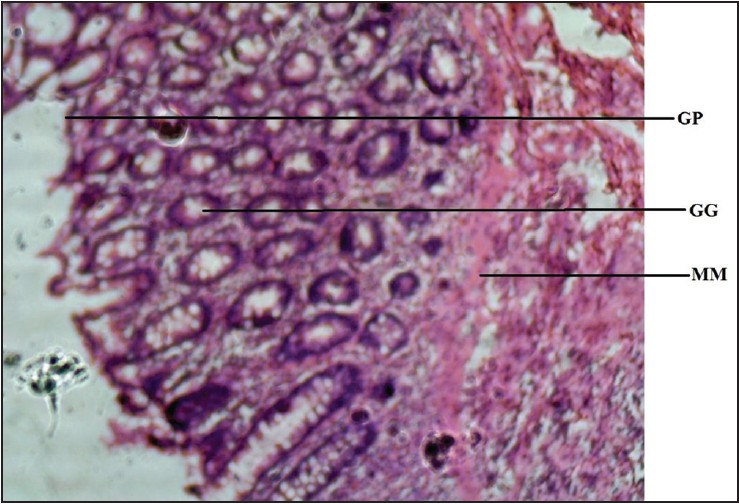 | Figure 2: Photomicrograph of rat stomach showing gastric gland (GG), gastric pit (GP), and muscularis mucosae (MM). Stained with H&E (100×)
Click here to view |
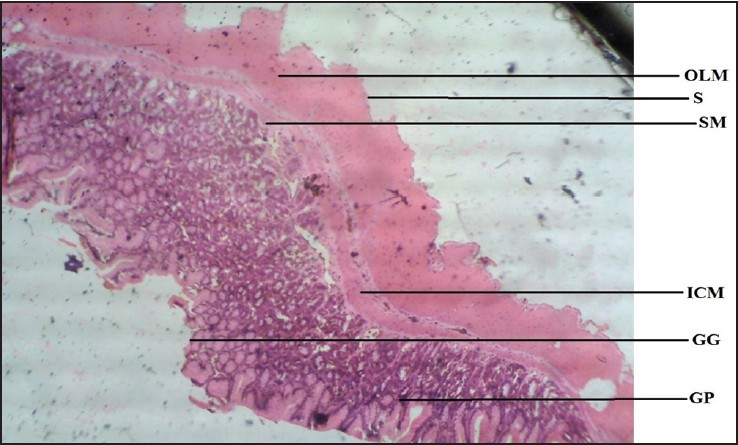 | Figure 3: Photomicrograph of agama lizard stomach showing gastric gland (GG), gastric pit (GP), submucosa (SM), inner circular muscle (ICM), outer longitudinal muscle (OLM), and serosa (S). Stained with H&E (40×)
Click here to view |
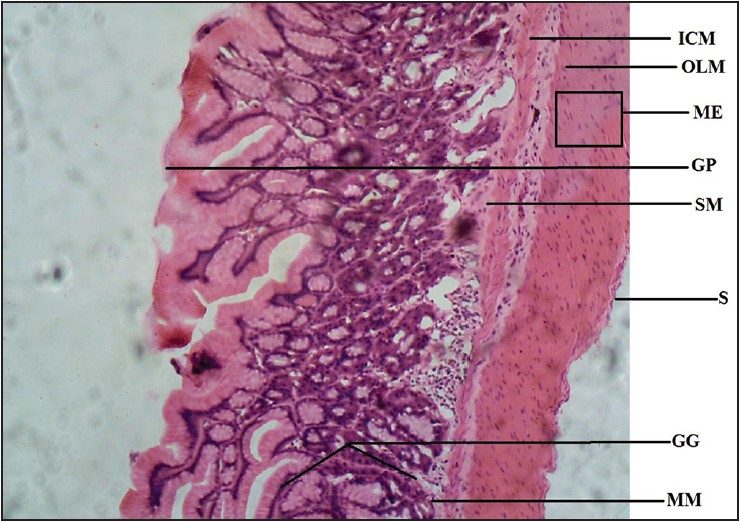 | Figure 4: Photomicrograph of agama lizard stomach showing gastric gland (GG), gastric pit (GP), muscularis mucosae (MM), submucosa (SM), muscularis externa (ME), inner circular muscle (ICM), outer longitudinal muscle (OLM), and serosa (S). Stained with H&E (100×)
Click here to view |
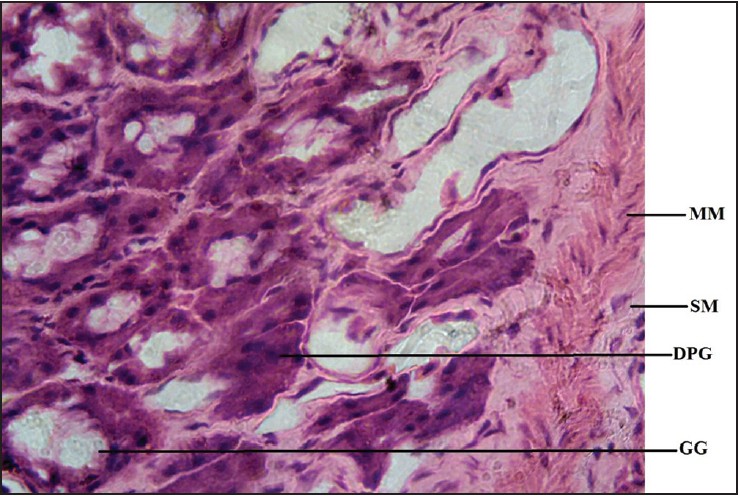 | Figure 5: Photomicrocraph of agama lizard stomach showing gastric gland (GG), muscularis mucosae (MM), submucosa (SM), and dark pigmented granule (DPG). Stained with H&E (400×)
Click here to view |
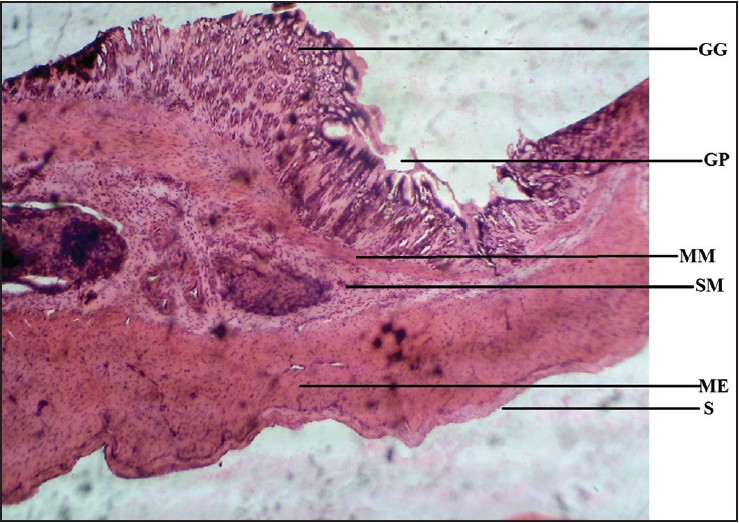 | Figure 6: Photomicrograph of cane toad stomach showing gastric gland (GG), gastric pit (GP), muscularis mucosae (MM), submucosa (SM), muscularis externa (ME), and serosa (S). Stained with H&E (40×)
Click here to view |
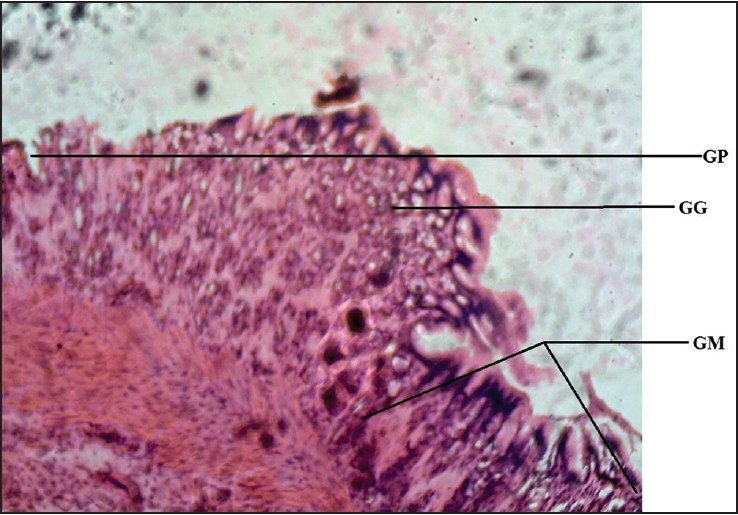 | Figure 7: Photomicrograph of cane toad stomach showing gastric gland (GG), gastric pit (GP), and gastric mucosa (GM). Stained with H&E (100×)
Click here to view |
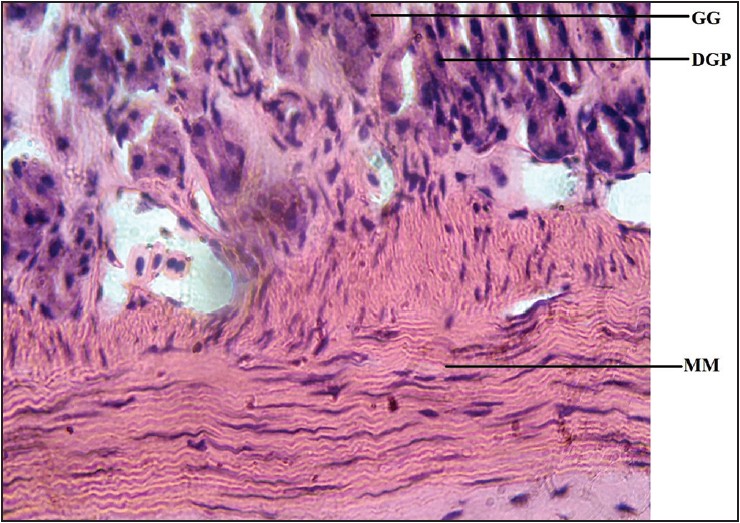 | Figure 8: Photomicrograph of cane toad stomach showing gastric gland (GG), muscularis mucosae (MM), dark pigmented granule (DPG). Stained with H&E (400×)
Click here to view |
The toad was found to have an extensive muscularis mucosae compared to other vertebrates. This finding suggests that there is an increase in the muscular activity of the smooth muscle fibers present in the muscularis mucosae and functioning to constantly keep the mucosal surface in contact with its gastric secretions. The gastric glands in Bufo marinus are relatively low and poorly visible. Hence it is a speculated increase in the activity of the smooth muscle fibers of the muscularis mucosae that compensates for the presence of only a few glands in the submucosa of the stomach, due to its relatively abundant lingual glands and their role in initiating digestion.
This finding varies from the findings from the rat's and lizard's stomachs. This in turn suggests a differential completion rate in the digestion of their diets. Since the toad and the lizard are carnivorous animals feeding mainly on insects, the rates of digestion vary with their respective gastric juices and the available total surface area of the food particle needed to make contact with the gastric secretions. It is believed that the rate of digestion is faster in the lizard than in the toad because it has an appreciably greater number of gastric glands.
Dark pigmented granules present in the gastric gland cells of some reptiles resembling mammalian pepsinogen granules, [7] were observed in the gastric mucosae of the stomachs of the lizards and, interestingly, in the toads as well. However, they were more numerous in the toads. These mammalian pepsinogen-like granules, when activated, account for the digestion of the proteins in the diets of the lizard and the toad, performing a somewhat similar function in mammals.
The muscularis externa was observed to be well defined in the rat's and the lizard's stomachs compared to the toad's. This provides the basis for peristalsis via the action of its inner circular and outer longitudinal layers at right angles to each other. [10] Therefore, during digestion, the churning action on the food by the stomach muscles leading to finer particles is more efficiently achieved in the lizard and rat. Consequently, this determines the surface area of the food particle that would be exposed to the gastric juice being secreted for the purpose of digestion as well as absorption. [11]
Amphibians possess simple columnar mucous cells lining the luminal surface and gastric pit of their fundic stomach. In our results, the Rattus novergicus showed mucus-secreting cells in its fundus and cardia. Romer et al. [12] stated that mammals are the only class of animals that has unique cardiac epithelium containing mucus-secreting glands. The gastric glands found in the Rattus novergicus were clearly distinguished from that of the others. This marked disparity is believed to be due to gradual wearing of the gastric mucosa in the Bufo marinus and in the Agama agama as well as the lack of a sufficient amount of bicarbonate-rich mucus-secreting cells in their fundic glands. It is thus believed that their insectivorous choices of diet contain a considerable amount of mucoid substances or mucus in their exoskeleton, in the central cells of their salivary gland as found in cockroaches and grasshoppers, and in their fat body cells. The mucus obtained from these feeds would help to substantiate the level of mucus produced by the fundic glands of their stomachs, in which mucus secretion alone may not adequately balance the level of acidity produced by the oxyntic cells of their glands and thus maintain a normal gastric pH level as needed for optimum activity of the gastric enzymes.
The stability in gastric pH of the stomach and overall rate of digestion in mammals would be less easily affected by choice of diet, since they possess mucus-secreting cells that help to maintain normal pH levels for optimum activity of gastric enzymes by balancing the level of acidity produced by the oxyntic cells of the stomach glands. This observation clinically suggests that the ultrastructure of a mammalian stomach should show little distortion in the event of a benign gastrinoma, with less spontaneity and severity of ulcer cases. Mammals possess a greater food storage capacity due to their ability to distend their muscular stomach walls. [1],[13]
From the physical examination of the stomach, the mammals were observed to possess the largest stomachs among the vertebrates under review. This finding could be linked to the greater food storage capacity of mammals.
| Conclusion and recommendations | |  |
Variations in size, shape, and histoarchitectural organization of the stomach make possible a useful interpretation regarding the relationship of stomach structure with diet. It suggests an adaptation to evolutionary advancement. This finding supports the view of Ofusori et al. [1] and Stevens and Humes [13] that differences in the diets of diverse species account for differences in the histomorphology of their stomachs. It is recommended that further studies be carried out to elucidate these findings.
| References | |  |
| 1. | Ofusori DA, Caxton-Martins EA, Keji ST, Oluwayinka PO, Abayomi TA, Ajayi SA. Microarchitectural adaptations in the stomach of African tree Pangolin ( Manis tricuspis). Int J Morphol 2008;26:701-5.  |
| 2. | Hildebrand M, Goslow G. Analysis of Vertebrate Structure. 5 th ed. New York: John Wiley & Sons Inc.; 2001. p. 201-17.  |
| 3. | Kahle H. Observation of gastric secretions in stomach of the Testudogreeca (Reptilia). J Exp Physiol 1913;152:129-30.  |
| 4. | Diefenbach CO. Gastric function in Caiman Crocodilus (Crocodylia; Reptilia). Comp Biochem Physiol 1975;51A:259-65.  |
| 5. | Langley JN. A physiological study on the presence of pepsin in relation to digestion in colubernatrix (Reptilia). J Exp Physiol 1881;172:66-7.  |
| 6. | Sancar-Bas S, Kaptan E, İnceli M, Sezen A, Us H. Glyconjugate histochemistry in the fundic stomach and small intestine of the frog ( Rana ridibunda). IUFS J Biol 2009;68:93-104.  |
| 7. | Smith DM, Tabin CJ. BMP signaling specifies the pyloric sphincter. Nature 1999;402;748-9.  |
| 8. | Ofusori DA, Caxton-martins EA. A comparative histomorphometric study on the stomach of rat ( Rattus norvegicus), bat ( Eidolon helvum) and pangolin ( Manis tricuspis) in relation to diet. Int J Morphol 2008;26:669-74.  |
| 9. | Sheehan DC, Hrapchak BB. Theory and Practice of Histotechnology. 2 nd ed. Columbus: Battelle Memorial Institute; 1987. p. 39-40.  |
| 10. | Young B, Lowe JS, Stevens A, Heath JW, Deakin DJ. Wheather's Functional Histology: A Text and Color Atlas. 5 th ed. Edinburg: Churchill Livingstone; 2006. p. 264-6.  |
| 11. | Liquori GE, Ferri D, Scillitani G. Fine structure of the oxynticopeptic cells in the gastric glands of the ruin lizard, podarcis sicula campestris De Betta, 1857 . J Morphol 2000;243:167-71.  |
| 12. | Romer AS, Parsons TS. The Vertebrate Body. Philadelphia: Saunders; 1962.  |
| 13. | Stevens CE, Hume ID. Comparative Vertebrate Physiology of the Vertebrate Digestive System. Cambridge: Cambridge University Press; 1995.  |
[Figure 1], [Figure 2], [Figure 3], [Figure 4], [Figure 5], [Figure 6], [Figure 7], [Figure 8]
|