|
 
 |
|
ORIGINAL ARTICLE |
|
|
|
| Year : 2011 | Volume
: 17
| Issue : 3 | Page : 152-156 |
| |
A six-nucleotide deletion polymorphism in the casp8 promoter is associated with reduced risk of esophageal and gastric cancers in Kashmir valley
Manzoor Ahmad Malik1, Showkat Ali Zargar2, Balraj Mittal1
1 Department of Genetics, Sanjay Gandhi Post Graduate Institute of Medical Sciences, Raebareilly Road, Lucknow, India
2 Department of Gastroenterology, Sher-I-Kashmir Institute of Medical Sciences, Srinagar, Kashmir, India
| Date of Web Publication | 20-Jan-2012 |
Correspondence Address:
Balraj Mittal
Department of Genetics, Sanjay Gandhi Post Graduate Institute of Medical Sciences, Raebareilly Road, Lucknow - 226 014
India
 Source of Support: None, Conflict of Interest: None  | 2 |
DOI: 10.4103/0971-6866.92090

 Abstract Abstract | | |
Background: Caspase-8 (CASP8) is a key regulator of apoptosis or programmed cell death, an essential defense mechanism against hyperproliferation and malignancy. To evaluate the role of CASP8 polymorphisms in esophageal (EC) and gastric cancers (GC) in the Kashmir valley, we examined the risk due to -652 6N ins/del polymorphism (rs3834129) in the promoter of CASP8 in a case-control study.
Materials and Methods: Genotypes of the CASP8 polymorphisms (-652 6N ins/del; rs3834129) were determined for 315 patients (135 EC and 108 GC) and 195 healthy controls by polymerase chain reaction. Data was statistically analyzed using Chi-square test and logistic regression model by using the SPSS software.
Results: Carriers for the "del" allele of rs3834129 single nucleotide polymorphism were associated with decreased risk for both EC (odds ratio [OR] = 0.278; 95% confidence interval [95% CI] = 0.090-0.853; P = 0.025) and GC (OR = 0.397; 95% CI = 0.164-0.962; P = 0.041). Also, in a recessive model, our results showed that CASP8 -652 6N ins/del "del/del" allele was conferring significant low risk for both EC (OR = 0.380; 95% CI = 0.161-0.896; P = 0.027) and GC (OR = 0.293; 95% CI = 0.098-0.879; P = 0.029). However, interaction of CASP8 -652 6N ins/del genotypes with smoking and high consumption of salted tea did not further modulate the risk of EC and GC.
Conclusions: Polymorphism in CASP8 -652 6N ins/del polymorphism modulates the risk of EC and GC in Kashmir valley.
Keywords: Caspase 8, esophageal and gastric cancer, Kashmir valley, polymorphism
How to cite this article:
Malik MA, Zargar SA, Mittal B. A six-nucleotide deletion polymorphism in the casp8 promoter is associated with reduced risk of esophageal and gastric cancers in Kashmir valley. Indian J Hum Genet 2011;17:152-6 |
How to cite this URL:
Malik MA, Zargar SA, Mittal B. A six-nucleotide deletion polymorphism in the casp8 promoter is associated with reduced risk of esophageal and gastric cancers in Kashmir valley. Indian J Hum Genet [serial online] 2011 [cited 2016 May 13];17:152-6. Available from: http://www.ijhg.com/text.asp?2011/17/3/152/92090 |
 Introduction Introduction | |  |
Among human cancers, esophageal and gastric carcinogenesis also appear to be a complex multistep processes with multi-functional etiologies, where environmental, geographical and genetic factors have been attributed to play major roles in the causation of the cancers. Esophageal cancer (EC) is the eighth and gastric cancer (GC) is the second most commonly occurring cancer in the world. [1] In India, EC and GC are the leading sites of tobacco-related cancers. Within the Indian subcontinent, the Valley of Kashmir presents a strikingly different picture, where the incidence of EC and GC have been reported to exceed 40% of all cancers, and the incidence is three- to six-times higher than that at various metropolis cancer registries in India. [2],[3] Some of the environment factors have been reported to be associated with an increased risk of EC and GC in Kashmir valley. [4] However, very few reports have associated this malignancy with specific risk factors prevalent in the area.
Apoptosis is an essential genetic program necessary for the proper development of an organism. [5] Thus far, two major apoptosis-signaling pathways have been described: extrinsic and intrinsic pathways. In both pathways, the initiator caspase, caspase-8 encoded by CASP8 (MIM: 601763) located at 2q33, plays an important role in transducing the death signal to more downstream death effectors such as caspase-3 and caspase-7. [6] In the extrinsic apoptosis pathway, caspase-8 triggers apoptosis caused mainly by death receptor-induced apoptotic signaling and mediated by Fas and Fas ligand. [7],[8] There are at least 353 single-nucleotide polymorphisms (SNP) of the CASP8 reported in the dbSNP database ( http://www.ncbi.nlm.nih.gov/ SNP/snp_ref.cgi? choose Rs=allandgo=GoandlocusId=841); however, only two common polymorphic variations, D302H (rs1045485) and 6N ins/del (rs3834129), have been reported to influence the risk of cancer development. [8],[9] The D302H polymorphism with unknown functionality seems extremely rare (minor allele frequency <1%) in Asian populations (based on the HapMap Project and the Environmental Genome Project databases). Therefore, in the current study, we investigated the roles of CASP8 -652 6N ins/del (rs3834129) polymorphisms in conferring genetic susceptibility to EC and GC in Kashmir valley.
 Materials and Methods Materials and Methods | |  |
The present case-control study comprised untreated histopathologically confirmed cases with EC (135), GC (108) and healthy controls (195). All subjects were unrelated permanent residents of Kashmir and were referred from the Departments of Gastroenterology, Sher-i-Kashmir Institute of Medical Sciences, Srinagar, from May 2006 to December 2008. Patients and controls were matched by ethnicity, mean age and gender. Patients were excluded if they had nonmalignant conditions like corrosive esophageal injury, achalasia injury, Barrett's esophagus, gastro-esophageal reflux disease (GERD) and nonulcer dyspepsia. Controls were also recruited from Sher-i-Kashmir Institute of Medical Sciences, Srinagar, which included medical staff as well as individuals who came for their routine checkup for conditions not related to cancer and were diagnosed as no severe ailments. All individuals were personally interviewed about their age, occupational history, medical history of other diseases, demographic features, family history of cancer, use of hot noon chai (salted tea), drinking alcohol and smoking habits. Tobacco use included smoking cigarettes or ''hukka'' (water pipe). Written informed consent was obtained from all study participants. The research protocol was approved by the ethics committee of Sanjay Gandhi Postgraduate Institute of Medical Sciences, Lucknow (project number: 5/13/48/ 2002-NCDIII). Sample collection, storage and transport were in compliance with committee guidelines. Blood samples were collected in EDTA and genomic DNA was extracted from peripheral blood leukocyte pellet using the standard salting-out method. [10] The quality and quantity of DNA was checked by agarose gel electrophoresis and spectrophotometry using a Nanodrop Analyser (ND-1000) (Nano Drop Technologies Inc., Wilmington, DE, USA).
Genotyping by polymerase chain reaction (PCR) and restriction fragment analysis
The CASP8 -652 6N ins/del polymorphism was determined using PCR. The details of the genotyping method used were same as that described previously. [11],[12] More than 15% of the samples were randomly selected for confirmation, and the results were 100% concordant.
Statistical analysis
Demographic characteristics of patients and controls were described as frequencies and percentages, whereas descriptive statistics of patients and controls were presented as mean and standard deviations for continuous measures. Statistical significance of frequency differences between patients and control groups was evaluated using the χ2 test. Deviation from the Hardy-Weinberg equilibrium in controls was assessed using the χ2 test; P-value was considered significant at the <0.05 level. Risk estimates were calculated for codominant, dominant and recessive genetic models using the most common homozygous genotype as reference. Observed genotype frequencies for CASP8 -652 6N ins/del polymorphism in controls were examined for deviation from the Hardy-Weinberg equilibrium (HWE) using a goodness-of-fit χ2 -test with one degree of freedom. The same controls were used for analyzing two sets of cancer cases. Binary logistic regression analysis was used to fit statistical models to predict the association of CASP8 -652 6N ins/del genotypes with susceptibility to EC and GC. Association was expressed as odds ratios (OR) for risk estimation with 95% confidence intervals (95% CI). All statistical analyses were performed using SPSS software version 15.0 (SPSS, Chicago, IL, USA).
 Results Results | |  |
Population characteristics: The mean age of healthy subjects (controls) and patients with EC and GC was 57.98 ± 12.664 years, 60.38 ± 8.402 years and 55.91 ± 9.728 years, respectively (t-test; P-value = ns). Both cancers were highly prevalent in males (68.1% in EC and 83.3% in GC) than in females. In patients with EC, squamous cell carcinoma (SCC) histopathology was common (76.3%), but in GC most of the cases were with adenocarcinoma (ADC, 79.6%). Smoking habit (Hukka) showed significantly higher risk both in EC (OR = 21.443; 95% CI = 11.628-39.543; P = 0.0001) and in GC (8.975; 95% CI = 5.156-15.622; P = 0.0001) patients. Individuals consumed salted-tea in a range of two to eight cups per day, and median consumption of tea was four cups per day. Therefore, we grouped individuals into ≤4 cups or >4 cups per day, and individuals consuming salted tea >4 cups per day were regarded as high salted tea consumers. Higher consumption of salted tea was also found to be associated with increased risk of EC (OR = 14.856; 95% CI = 8.411-26.241; P-value = 0.0001) and GC (OR = 14.778; 95% CI = 8.020-27.231; P-value = 0.0001) [Table 1]. None of the patients or controls reported consumption of alcohol and, therefore, interaction of alcohol intake with genetic variations could not be analyzed. Other dietary factors like consumption of "Haak" and "Wur" were not found to be associated with EC or GC development (data not shown).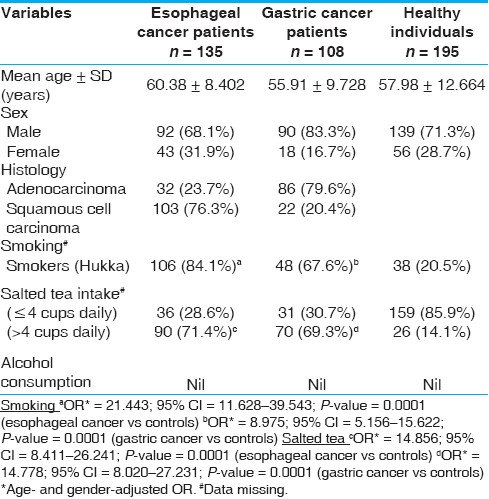 | Table 1: Characteristics of esophageal, gastric cancer patients and healthy individuals of the Kashmir valley
Click here to view |
Association between CASP8 -652 6N ins/del polymorphism and the EC and GC risk: The genotype and allele frequencies of the CASP8 polymorphisms among cases and controls are shown in [Table 2]. The observed genotype frequencies among the control subjects were in agreement with the Hardy-Weinberg equilibrium (P = 0.127; χ2 = 2.332). In the present study, when we used the CASP8 -652 6N ins/ins genotype as the reference, we found that the CASP8 -652 6N del/del genotype was significantly associated with low risk in EC (OR = 0.278; 95% CI = 0.090-0.853; P-value = 0.025) as well as with GC (OR = 0.397; 95% CI = 0.164-0.962; P-value = 0.041), respectively. Furthermore, a significant decreased risk of EC and GC was found with the CASP8 -652 (del/del genotype) compared with the -652 6N ins/del + del/del genotypes, suggesting a recessive protective effect of this polymorphism on both EC (OR = 0.380; 95% CI = 0.161-0.896; P-value = 0.027) and GC (adjusted OR = 0.293; 95% CI = 0.098-0.879; P-value = 0.029). | Table 2: Frequency distribution of CASP8 -652 6N ins/del and risk assessment in esophageal and gastric cancer patients and controls
Click here to view |
Interaction of CASP8 -652 6N ins/del genotypes with smoking habit and high salted tea consumption: Our results also show a significant association of smoking (Hukka) and high consumption of salted tea with EC and GC [Table 1]. However, in gene environmental interaction, we did not find any significant association while analyzing the CASP8 -652 6N ins/del genotypes with smoking [Table 3] and high salted tea consumption [Table 4].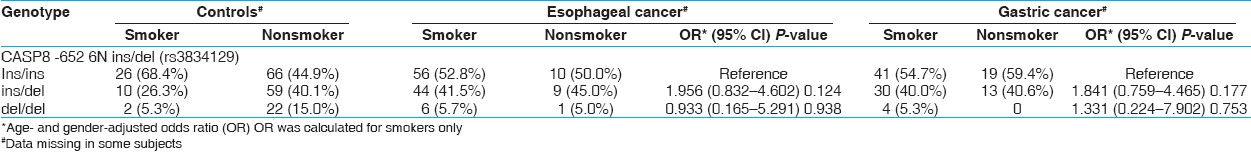 | Table 3: Interaction of CASP8 -652 6N ins/del genotypes and smoking in modulation of EC and GC risk
Click here to view |
 | Table 4: Interaction of CASP8 -652 6N ins/del genotypes and salted tea in modulation of EC and GC risk
Click here to view |
 Discussion Discussion | |  |
Apoptosis or programmed cell death is a crucial mechanism against hyperproliferation and malignancy. [13] Caspases are a family of highly conserved intracellular aspartate-specific cysteine proteases that are key intermediaries of the apoptotic process. [14] Studies in a range of human cancers, such as Hodgkin lymphoma, gastric carcinoma and head and neck cancer, have established the role of somatic mutations in CASP genes, which represses apoptosis, leading to illegitimate cell proliferation and anomalous cell survival. [15],[16],[17] These observations provide compelling evidence that low-penetrance genetic variations in CASP genes could also play a substantial role in modifying the risk for various cancers.
In the present study, we found that the CASP8 -652 6N del/del genotypes were associated with a significantly decreased EC and GC risk compared with the ins/ins genotype, which is inconsistent with the findings in several types of cancers (lung, esophageal, stomach, colorectal, breast and cervical cancers). [18],[19]
The 6-bp ins/del polymorphism (-652 6N ins/del; rs3834129) is located in the promoter region of the CASP8 gene and eliminates a Sp1 transcription factor binding site. This results in decreased RNA transcription in lymphocytes, and lower CASP8 activity. [18] Therefore, a possible mechanism underlying the CASP8 polymorphism associated in the decreased risk in EC and GC in our study is that this polymorphism may reduce apoptotic potential in the T lymphocyte and make malignant cells less likely escape from CTL killing, ultimately protecting against EC and GC. [18]
The people of the valley have many unique dietary features that are different from the rest of the world. Salted tea used by people is prepared by using baking soda (sodium bicarbonate) along with common salt (sodium chloride) and boiled for a few hours before consumption. It has been suspected that the salts might cause thermal injury to the esophageal and gastric epithelium. [2] In the present study, high consumption of salted tea (>4 cups a day) was independently associated with increased risk for EC (OR = 14.856; 95% CI = 8.411-26.241; P-value = 0.0001) and GC (OR = 14.778; 95% CI = 8.020-27.231; P-value = 0.0001). Our results also show a significant association of smoking (Hukka) with EC (OR = 21.443; P-value = 0.0001) and GC (OR = 8.975; P-value = 0.0001). However, based on our gene environmental interactions, we did not find any significant association of CASP8 -652 6N ins/del genotypes with smoking or high salted tea consumption.
The limitation in our study was smaller sample size. As it is the first report of genetic susceptibility of EC and GC due to CASP8 -652 6N ins/del polymorphisms in the Kashmiri valley, there is a definite need to perform a similar study in a larger sample size before its clinical application.
In conclusion, the present case-control study found that CASP8 -652 6N ins/del polymorphism was associated with reduced risk for EC and GC risk in the Kashmir valley. High salted tea intake and smoking itself are risks for developing EC and GC, but the risk was not further enhanced due to interaction of genetic variants analyzed in the present study.
 References References | |  |
| 1. | Parkin DM. The role of cancer registries in cancer control. Int J Clin Oncol 2008;13:102-11. 
[PUBMED] [FULLTEXT] |
| 2. | Khuroo MS, Zargar SA, Mahajan R, Banday MA. High incidence of oesophageal and gastric cancer in Kashmir in a population with special personal and dietary habits. Gut 2009;33:11-5. 
|
| 3. | Siddiqiz M, Tricker AR, Preussmann R. The occurrence of preformed N nitroso compounds in food samples from a high risk area of esophageal cancer in Kashmir, India. Cancer Lett 1988;39:37-43. 
|
| 4. | Siddiqi M, Kumar R, Fazili Z, Spiegelhalder B, Preussmann R. Increased exposure to dietary amines and nitrate in a population at high risk of oesophageal and gastric cancer in Kashmir (India). Carcinogenesis 1992;13:1331-5. 
[PUBMED] [FULLTEXT] |
| 5. | Opferman JT. Apoptosis in the development of the immune system. Cell Death Differ 2008;15:234-42. 
[PUBMED] [FULLTEXT] |
| 6. | Heikaus S, Kempf T, Mahotka C, Gabbert HE, Ramp U. Caspase-8 and its inhibitors in RCCs in vivo: The prominent role of ARC. Apoptosis 2008;13:938-49. 
[PUBMED] [FULLTEXT] |
| 7. | Wang S, El-Deiry WS. TRAIL and apoptosis induction by TNF-family death receptors. Oncogene 2003;22:8628-33. 
[PUBMED] [FULLTEXT] |
| 8. | Juo P, Kuo CJ, Yuan J, Blenis J. Essential requirement forcaspase-8/FLICE in the initiation of the Fas-induced apoptotic cascade. Curr Biol 1998;8:1001-8. 
[PUBMED] [FULLTEXT] |
| 9. | Li C, Zhao H, Hu Z, Liu Z, Wang LE, Gershenwald JE, et al. Genetic variants and haplotypes of the caspase-8 and caspase-10 genes contribute to susceptibility to cutaneous melanoma. Hum Mutat 2008;29:1443-51. 
[PUBMED] [FULLTEXT] |
| 10. | Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res 1988;16:1215. 
[PUBMED] [FULLTEXT] |
| 11. | Son JW, Kang HK, Chae MH, Choi JE, Park JM, Lee WK, et al. Polymorphisms in the caspase-8 gene and the risk of lung cancer. Cancer Genet Cytogenet 2006;169:121-7. 
[PUBMED] [FULLTEXT] |
| 12. | Hu Z, Li C, Chen K, Wang LE, Sturgis EM, Spitz MR, et al. Single nucleotide polymorphisms in selected apoptotic genes and BPDE-induced apoptotic capacity in apparently normal primary lymphocytes: A genotype-phenotype correlation analysis. J Cancer Epidemiol 2008;2008:14705. 
|
| 13. | Hengartner MO. The biochemistry of apoptosis. Nature 2000;407:770-6. 
[PUBMED] [FULLTEXT] |
| 14. | Siegel RM. Caspases at the crossroads of immune cell life and death. Nat Rev Immunol 2006;6:308-17. 
[PUBMED] [FULLTEXT] |
| 15. | Mandruzzato S, Brasseur F, Andry G, Boon T, van der Bruggen P. A CASP-8 mutation recognized by cytolytic T lymphocytes on a human head and neck carcinoma. J Exp Med 1997;186:785-93. 
[PUBMED] [FULLTEXT] |
| 16. | Shin MS, Kim HS, Kang CS, Park WS, Kim SY, Lee SN, et al. Inactivating mutations of CASP10 gene in non-Hodgkin lymphomas. Blood 2002;99:4094-9. 
[PUBMED] [FULLTEXT] |
| 17. | Soung YH, Lee JW, Kim SY, Jang J, Park YG, Park WS, et al. CASPASE-8 gene is inactivated by somatic mutations in gastric carcinomas. Cancer Res 2005;65:815-21. 
[PUBMED] [FULLTEXT] |
| 18. | Sun T, Gao Y, Tan W, Ma S, Shi Y, Yao J, et al. A six-nucleotide insertion-deletion polymorphism in the CASP8 promoter is associated with susceptibility to multiple cancers. Nat Genet 2007;39:605-13. 
[PUBMED] [FULLTEXT] |
| 19. | Frank B, Rigas SH, Bermejo JL, Wiestler M, Wagner K, Hemminki K, et al. The CASP8 -652 6N del promoter polymorphism and breast cancer risk: A multicenter study. Breast Cancer Res Treat 2008;111:139-44. 
[PUBMED] [FULLTEXT] |
[Table 1], [Table 2], [Table 3], [Table 4]
|