|
 
 |
|
REVIEW ARTICLE |
|
|
|
| Year : 2014 | Volume
: 20
| Issue : 2 | Page : 129-141 |
| |
Mammalian non-classical major histocompatibility complex I and its receptors: Important contexts of gene, evolution, and immunity
BM Pratheek1, Tapas K Nayak1, Subhransu S Sahoo1, Prafulla K Mohanty2, Soma Chattopadhyay3, Ntiya G Chakraborty4, Subhasis Chattopadhyay1
1 School of Biological Sciences, National Institute of Science Education and Research, Bhubaneswar, Odisha, India
2 Department of Zoology, Utkal University, Bhubaneswar, Odisha, India
3 Infectious Disease Biology, Institute of Life Sciences, Bhubaneswar, Odisha, India
4 Department of Medicine, University of Connecticut Health Center, Farmington, USA
| Date of Web Publication | 14-Oct-2014 |
Correspondence Address:
Ntiya G Chakraborty
Department of Medicine, University of Connecticut Health Center, Farmington
USA
Soma Chattopadhyay
Infectious Disease Biology, Institute of Life Sciences, Bhubaneswar, Odisha
India
Subhasis Chattopadhyay
School of Biological Sciences, National Institute of Science Education and Research, Bhubaneswar, Odisha
India
 Source of Support: The work was partly supported by Department of Biotechnology, Ministry of Science and Technology, Govt. of India (Project no: T/PR13312/GBD/27/247/2009); (BT/PR13118/GBD/27/186/2009) and by Council of Scientific and Industrial Research (CSIR) Project No. 37 (1542)/12/EMR-II), Ministry of Science and Technology, Govt. of India,, Conflict of Interest: None
DOI: 10.4103/0971-6866.142855

 Abstract Abstract | | |
The evolutionary conserved, less-polymorphic, nonclassical major histocompatibility complex (MHC) class I molecules: Qa-1 and its human homologue human leukocyte antigen-E (HLA-E) along with HLA-F, G and H cross-talk with the T-cell receptors and also interact with natural killer T-cells and other lymphocytes. Moreover, these nonclassical MHC molecules are known to interact with CD94/NKG2 heterodimeric receptors to induce immune responses and immune regulations. This dual role of Qa-1/HLA-E in terms of innate and adaptive immunity makes them more interesting. This review highlights the new updates of the mammalian nonclassical MHC-I molecules in terms of their gene organization, evolutionary perspective and their role in immunity.
Keywords: CD94/NKG2, human leukocyte antigen-E, major histocompatibility complex, Qa-1
How to cite this article:
Pratheek B M, Nayak TK, Sahoo SS, Mohanty PK, Chattopadhyay S, Chakraborty NG, Chattopadhyay S. Mammalian non-classical major histocompatibility complex I and its receptors: Important contexts of gene, evolution, and immunity. Indian J Hum Genet 2014;20:129-41 |
How to cite this URL:
Pratheek B M, Nayak TK, Sahoo SS, Mohanty PK, Chattopadhyay S, Chakraborty NG, Chattopadhyay S. Mammalian non-classical major histocompatibility complex I and its receptors: Important contexts of gene, evolution, and immunity. Indian J Hum Genet [serial online] 2014 [cited 2016 Aug 23];20:129-41. Available from: http://www.ijhg.com/text.asp?2014/20/2/129/142855 |
 Introduction Introduction | |  |
Major histocompatibility complex class I molecules (MHC-I) are cell surface glycoproteins expressed on most of the cells. On antigen presenting cells, they are involved in the presentation of endogenous peptide to CD8 + T-cells through T-cell receptors (TCR) for the antigens that are originated from the cytosolic protein by proteasomal degradation. Nevertheless, MHC-I molecules also present peptides, which are generated from exogenous proteins by a process called cross-presentation. [1] In humans, MHC-I proteins are encoded by (a) highly polymorphic classical MHC class Ia and (b) less-polymorphic nonclassical MHC class Ib genes. Classical MHC-Is are human leukocyte antigen (HLA)-A, -B and -C. On the other hand, human nonclassical MHC-Is are HLA-E, -F, -G, and -H (also called "High Fe" or HFE), which are homologous to Qa-1, Qa-2, HFE and RT1 haplotypes in mouse and rat, respectively. [2],[3],[4],[5],[6],[7] In this review, we briefly describe the gene organization, a phylogenetic analysis of nonclassical MHC molecules and updates on their immunological interaction with receptors like TCR and CD94/NKG2 on T, NK and natural killer T (NKT) cells. We also discuss their role in the pathological state of some important diseases that are associated with altered host cell immunity, which has implication in the basic and translational research of mammalian immune responses and their regulation.
 Gene Organization and Evolutionary Perspective of Nonclassical Major Histocompatibility Complex Class I Molecules Gene Organization and Evolutionary Perspective of Nonclassical Major Histocompatibility Complex Class I Molecules | |  |
Genes of nonclassical MHC-I are located in chromosome 6 (locus p21.1-21.3) in humans. [8] However, in mice and rats they are found in chromosome 17 (locus B1) and 20 (locus p12), respectively [Figure 1]. [9],[10] Classical MHC-I molecules and nonclassical MHC-I are expressed in most of the tissues in modest levels, but these are expressed in high quantities in some neoplastic cells. [11],[12],[13],[14] Nonclassical MHC-Is are known to be evolutionary conserved.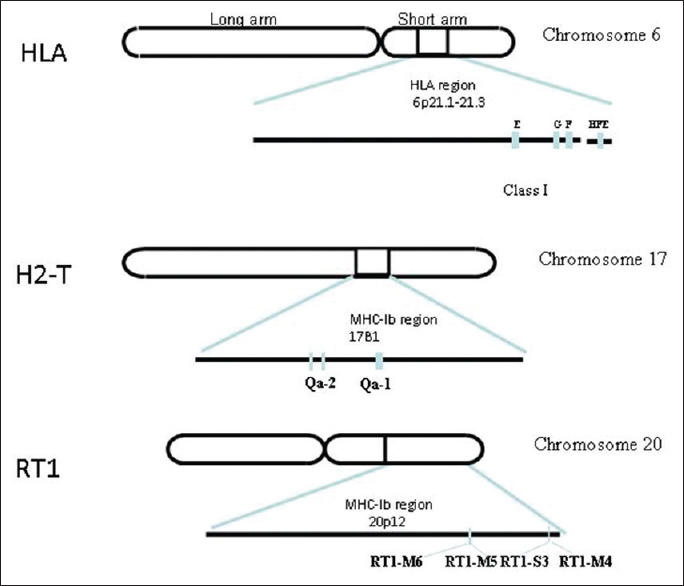 | Figure 1: Schematic representation of nonclassical major histocompatibility complex (MHC) class I genes of human, mouse and rat: Nonclassical MHC class I genes found in human, mouse, rat in chromosome 6, 17 and 20 respectively. The nonclassical MHC-I genes of human leukocyte antigen (HLA-E,-F,-G,-H.) are found in locus p21.1, mouse (Qa-1, Qa-2) are found in B1 region, rat (RT1-M4,-M5,-M6,-S3) are found in region 20p12 of chromosome 20
Click here to view |
It has been suggested from numerous studies that two alleles of HLA-E (HLA-E*0101 and HLA-E*0103) have minimum polymorphism among all HLAs and are found in high frequencies in Caucasians. [15],[16] Among these two alleles, HLA-E*0101 (also known as HLA-E 107R ) is expressed strongly in normal cells and in higher frequency than HLA-E*0103 (HLA-E 107G ). HLA-E*0101 differs from HLA-E*0103 at amino acid position 107 where arginine is replaced by glycine. [16]
Mouse MHC complex is known as H-2 complex located on chromosome 17. [6],[10] In mice several MHC-Ibs are found in the H-2Q, H-2T, and H-2M regions of MHC, whereas Qa-1b is a mouse MHC-Ib molecule encoded by the T23 gene. [17] It was first identified in peripheral T-cells as a serological determinant and later identified as the previously isolated gene 37. [17] Sequence analysis suggests that there are only four known alleles of Qa-1. Qa-1b is expressed in the majority of inbred laboratory strains, whereas Qa-1a is expressed in most of other strains. On the other hand, Qa-1c and Qa-1d frequencies are very rare. [18]
The ability to induce an allogeneic immune response by Qa-1 defines the function of Qa-1 as an MHC ligand for T-cells, which is not restricted by H-2D or H-2K haplotype. [19] An investigation by Aldrich et al. [20] decipher the cell surface expression of the Qa-1 alloantigens with the help of monoclonal anti-Qa-1 cytotoxic T lymphocyte (CTL) cell lines. It has been found that the expression of Qa-1 is high, similar to class I H-2K/D molecules. Moreover, the Qa-1 determinant modifier (Qdm) has been found to be linked with H-2D gene. It is also observed that Qdm may control over expression of certain CTLs-defined Qa-1 antigenic determinants. [20] Another report also suggests that a majority of alloreactive Qa-1-specific CTL clones recognize a specific Qa-1 bound peptide, which is a derivative leader sequence of H-2D. [21] Several studies in the recent past suggest a key role of Qa-1 in innate immunity. Qa-1 is also reported as a ligand for CD94/NKG2 receptors in mouse NK cells, NKT cells and some subset of T-cells. [22],[23],[24],[25],[26] Accordingly, it appears that other than interaction with TCR; the nonclassical MHCs are important for signaling through CD94/NKG2 receptors to modulate host cell immunity. The role of CD94/NKG2 receptors in Qa-1 and HLA-E mediated immune responses is discussed in a subsequent section.
In several nonhuman primates, existence of MHC-Ib has been suggested earlier. MHC-G has been described in some nonhuman primates. [27],[28],[29],[30],[31] It has been mentioned that in chimpanzee (Pan troglodytes) MHC-Ib is known to be organized in similar way as human MHC-Ib. [32] It has also been described in case of many other nonhuman primate species. [27],[32],[33],[34],[35],[36],[37] MHC-I genes of New World primates appear to be homologous to HLA-G genes than classical HLA-I genes. [27],[28] Mamu-G is ortholog of HLA-G in the rhesus monkey (Macaca mulatta) and it is appeared to be a pseudogene. Another nonclassical MHC-I locus called Mamu-AG is also found to be expressed in the placenta of rhesus monkeys. Mamu-AG encodes MHC-IA locus-related molecules with all the features of human HLA-G, apart from features like a truncated cytoplasmic domain and limited polymorphism. [31] Phylogenetic study comprising exon 2, exon 3, and intron 2 sequences of MHC-G of 7 nonhuman primates along with HLA-G have shown that cotton top tamarin (Saguinus oedipus) MHC-G sequences are more closer to human and great apes (Pongids).[30]
HLA-E and-F homologues have been described in orangutans and macaques. [36],[37],[38],[39] The orthologs of MHC-E have also been identified in nonhuman primates such as gorillas, chimpanzees, bonobos, and vervet (green) monkeys. [38],[40] Phylogenetic analysis of MHC-E locus of six New World monkey species and full-length MHC-E cDNAs of four unrelated cotton-top tamarins (S. oedipus) along with HLA-E have shown that Saoe*01 (S. oedipus) is orthologous to HLA-E. [35] Moreover, multiple sequence alignment of MHC-F cDNA sequences of human, chimpanzee, macaque and cotton-top tamarin have shown that in cotton-top tamarin, accumulation of nonsynonymous differences are more than synonymous differences in the peptide binding region of this gene. [37] Analysis of the nucleotide sequences of MHC-H in gorillas and chimpanzees revealed that they have a high degree of homology among their alleles. [41] Phylogenetic analysis of some MHC-I genes of gorilla and chimpanzee along with human, shows the close clustering of Gogo-H*01 (gorilla) and Patr-H*01 (chimpanzee) with HLA-H alleles, indicating close evolutionary relationship between them. [41]
The gene and protein sequences [Figure 2] and [Table 1] and [Table 2] of nonclassical MHC-I molecules of rat, mouse, nonhuman primates (Gorilla gorilla, P. troglodytes, M. mulatta) and human have been analyzed by web based Clustal W 2.1 tool from DNA Data bank of Japan (DDBJ) with Unweighted Pair Group Method with Arithmetic Mean, 1000 bootstra P value (http://clustalw.ddbj.nig.ac.jp/). The gene sequence analysis of MHC-F of G. gorilla and protein sequence analysis of HLA-H or HFE and MHC-E are not included due to unavailability of proper sequences. It has been observed that nonclassical MHC-I molecules are clustered according to the different types. The protein sequences of nonhuman primates and human have shown maximum homology in case of MHC-G and MHC-F (MHC-G-98-99%, MHC-F-93-98%) whereas they are less conserved in case of MHC-H or HFE and MHC-E (MHC-E-57-64%, HFE or MHC-H-34-35%). Mouse and rat protein sequences are showing maximum identity only in HFE or MHC-H (87%), but for other types of nonclassical MHC-I molecules, they are showing around 50% identity (MHC-G-41-51%, MHC-E-51-53%). Protein sequences of human and nonhuman primates have revealed around 55% homology with rat and mouse and in case of all nonclassical MHC-I molecules (MHC-G-48-50%, MHC-E-56-57%, MHC-F-56-57%). Similar type of observation have been noticed in phylogenetic analyses in earlier studies of MHC-F and MHC-G of human and nonhuman primates. [30],[37] In addition, gene sequences analysis of nonclassical MHC-I revealed that there are around 34-90% similarity in case of MHC-G, 53-98% similarity for MHC-E, whereas among MHC-F and MHC-H or HFE have 48-87% and 54-98% similarity, respectively. Similar observation has been reported for MHC-H gene of human and nonhuman primates. [41]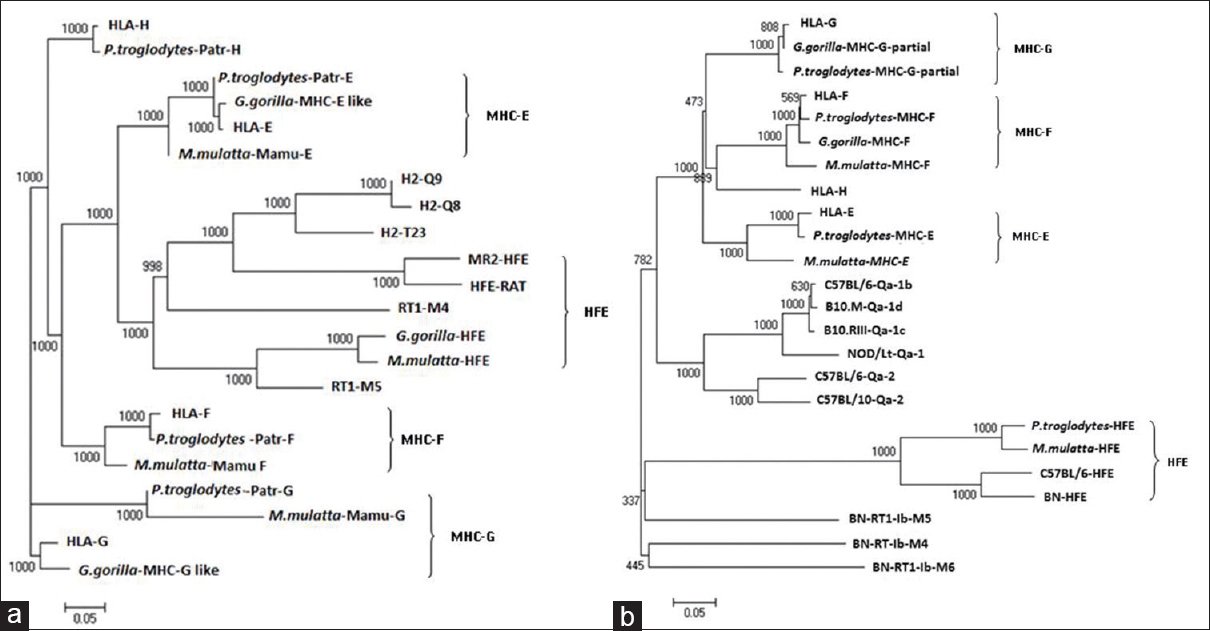 | Figure 2: Phylogenetic analysis of some of the sequences of genes and proteins of nonclassical major histocompatibility complex -I(MHC-I) of the human leukocyte antigen-I (HLA-I), nonhuman primates, rat (RT1) and mouse (Qa) with respective mouse and rat strains. Nonclassical MHC-I molecules showed that these are clustered according to types of non-classical MHC-I molecules. Phylogentic tree is constructed by Unweighted Pair Group Method with Arithmetic Mean method as implemented by Clustal w (DDBJ), Bootstra P value (1000 replicates) are indicated. (a) Nucleotide sequences of the genes included are: HLA-E (Gene ID: 3133):, HLA-G (GENE ID:3135), HLA-F (GENE ID:3134):, HLA-H (GENE ID:3136), H-Q9 (C57BL/6, GENE ID: 110558), H2-Q8 (C57BL/10, GENE ID: 15019), H2-T23 (C57BL/6,GENE ID: 15040), MR2-HFE (C57BL/6, GENE ID: 15216), RT1-M5 (BN, GENE ID:499400), RT1-M4 (BN, GENE ID: 309584), HFE (BN, GENE ID: 29199), Mamu-E (Macaca mulatta, GENE ID: 711532), Mamu-F (M. mulatta, GENE ID: 709076), Mamu-G (M. mulatta, GENE ID: 697260), HFE (M. mulatta, GENE ID: 696129), Patr-F (Pan troglodytes, GENE ID: 100169977), Patr-E (P. troglodytes, GENE ID: 462540), Patr-G (P. troglodytes, GENE ID: 494187), Patr-H(P. troglodytes, GENE ID: 741554) MHC-G-like (Gorilla gorilla GENE ID: 101143843), MHC-E-like (G. gorilla GENE ID: 101153360), HFE (G. gorilla GENE ID: 101126285). (b) Protein sequence from Genbank included in the analyses have the following accession numbers: HLA-E: BAB63328, HLA-G: BAB63336.1, HLA-F: ABD38924, HLA-H: P01893, Qa-2 (C57BL/6): AAX98170, Qa-2 (C57BL/10): AAB41657, Qa-1b (C57BL/6): NP_034528, Qa-1 (NOD/Lt mice): AAD53968, Qa-1c (B10.RIII): AAD12244.1, Qa-1d (B10.M): AAD31381, HFE (C57BL/6): NP_034554, RT1-M6(BN): NP_001008852, RT1-M4(BN): NP_001161815, RT1-M5(BN): NP_001161825, HFE(BN): NP_445753, MHC-G-partial (G. gorilla): AAL40082, MHC-F (G. gorilla): AAQ13398, Patr-E (P. troglodytes): NP_001038963, MHC-G-partial (P. troglodytes): AAK08128, MHC-F (P. troglodytes): AAQ13481, HFE (P. troglodytes): NP_001009101, MHC-E (M. mulatta): NP_001108438, MHC-F (M. mulatta): ABD38925, HFE (M. mulatta): NP_001247505
Click here to view |
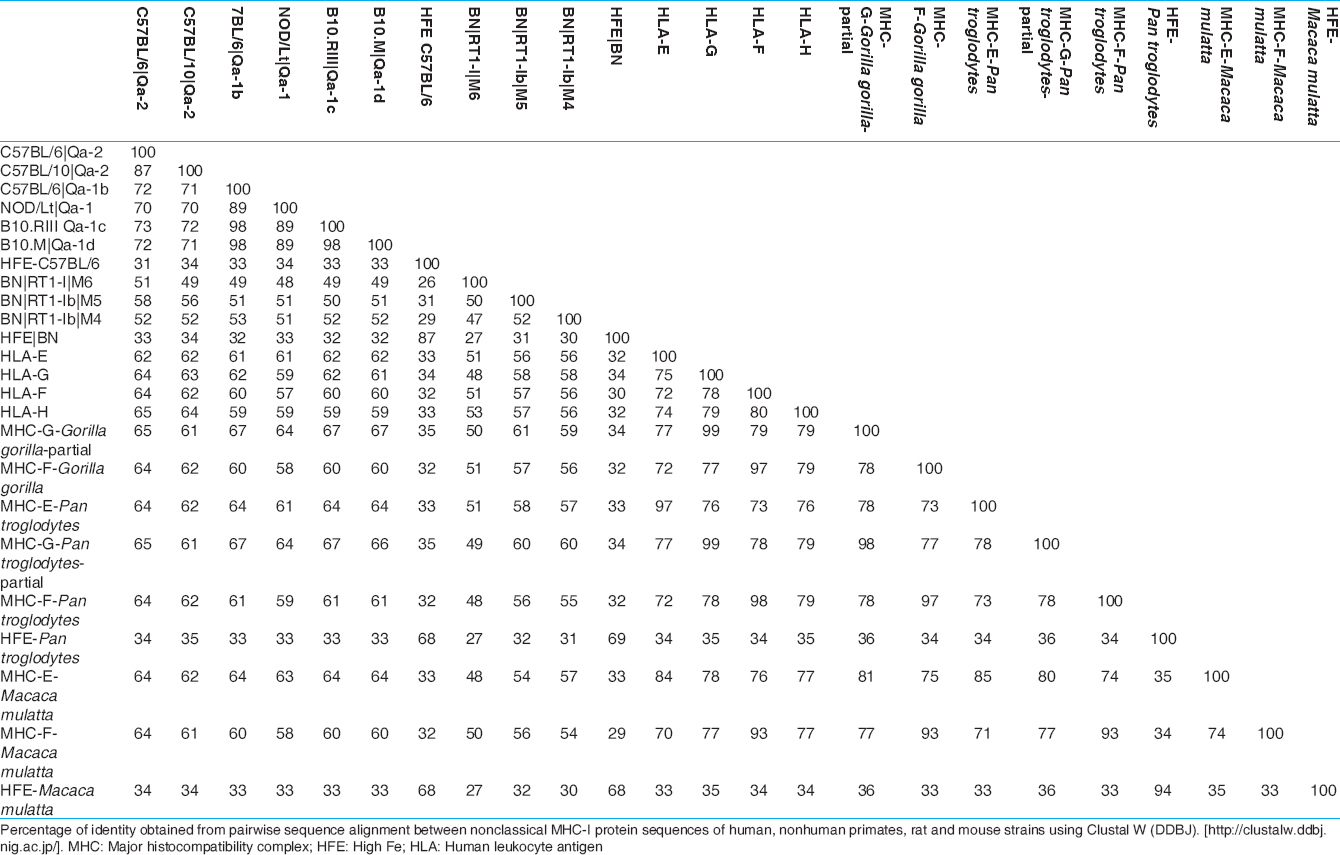 | Table 2: Similarity matrix of some nonclassical MHC - I protein sequences
Click here to view |
Moreover, it has been observed that human and nonhuman primates share maximum homology among each other for most of the nonclassical MHC-I genes (MHC-E-86-98%, MHC-F-72-87%, MHC-H or HFE-71-98%) as compared to other species [Figure 2]a and [Table 1].
 Involvement of Qa-1/HLA-E and CD94/NKG2 System in Altered Immunity and Diseases Involvement of Qa-1/HLA-E and CD94/NKG2 System in Altered Immunity and Diseases | |  |
Cellular and molecular basis of Qa-1/HLA-E and CD94/NKG2 system
HLA-E is found to be a ligand for CD94/NKG2A, B and C receptors on NK cells. [42] Moreover, it has been shown that CD94/NKG2A receptor expresses on CD8 + T-cells to induce immune inhibitory effect. [26] NK cells in mouse and human express heteromeric C-type lectin receptors comprising CD94 and NKG2. The NKG2A isoform is expressed more than other isoforms and has immunoreceptor tyrosine-based inhibitory motifs in its cytoplasmic domain, which form heterodimer with CD94 to inhibit NK cell function. [25],[43],[44]
CD94/NKG2C, an activating NK cell receptor of the C-type lectin superfamily, has been found to bind to HLA-E. Moreover, it noncovalently associates with DNAX-activation protein 12 (DAP12), a membrane receptor containing an immunoreceptor tyrosine-based activating motif (ITAM). [45] NK cells are found to recognize and destroy infected cells through Qa-1/HLA-E and CD94/NKG2 receptors. This "missing-self" phenomenon of NK cells plays a key role in recognizing and destroying abnormal cells. These attributes may facilitate viruses to acquire an important immune escape mechanism deviating host protective immunity. [46],[47]
Receptor profile of Qa-1/HLA-E and CD94/NKG2 system
Evidences in the recent past suggest that HLA-E has a role in restricting the αβ TCR bearing subsets of T-cells. [48],[49] Qa-1 and HLA-E are functional homologues, which are known to have an exclusive role in the regulation of NK cells. Moreover, it has been found that NKT cells co-express TCR and NK1.1 receptors. [50],[51],[52],[53]
Mouse invariant NKT (iNKT) cells that express NK cell receptors and TCR α chain of Vα14Jα18 (Vα24Jα15 in humans) and a semi variant TCR-β, which are found to be associated with Vβ8 (Vβ11 in humans), Vβ2 and Vβ7 receptors. [50],[51],[52] Vα14 TCR recognizes glycolipid antigens, such as α-galactosylceramide and its analogues presented on MHC-I like molecule CD1d. [51],[52],[54],[55],[56],[57],[58] iNKT cells are also known to be associated with CD94/NKG2 receptor subsets for their immunoregulatory role in mammalian immunity. [59] It has been shown that differential co-stimulatory signals can be mediated through CD80/86 and CD40 in antigen-presenting cells interacting with NKT cells expressing CD28 and CD154 respectively. [60] Moreover, these results suggest that CD28-CD80/CD86 and CD40-CD154 co-stimulatory pathways may differentially contribute to regulate Th1 and Th2 associated responses of NKT cells in vivo. However, the specific role of NKT cells in association to CD94/NKG2 and co-stimulatory responses needs further investigation.
Involvement of Qa-1/HLA-E and CD94/NKG2 system in autoimmune diseases
It has been suggested that induction of immunosuppressive CD8 + T-cells may be restricted by MH-Ib/Qa-1 to regulate CD4 + T-cell response. [61],[62] Moreover, most of the MHC class Ib molecules along with β2 microglobulin (β2m ) molecules are known to have interaction with CD8 co-receptors. TCR mediated suppression of CD4 + T-cell response by Qa-1 restricted CD8 + Treg cells has been demonstrated in an autoimmunity mice model of experimental autoimmune encephalomyelitis (EAE). [63] Moreover, it has been shown that the Qa-1-CD94/NKG2A mediated CD8 + Treg cell activity or activation may lead to complete restriction of EAE development. It has been shown that Qa-1 restricted a specific population of CD8αα+ Tregs can regulate EAE antigen-specific Vβ8.2 + CD4 + T-cell response. [64]
High CD94/NKG2A expression by T-cells has been demonstrated in remission patients following tumor necrosis factor (TNF) based TNF inhibitor therapy compared to active rheumatoid arthritis. Low CD94/NKG2A expression has been associated with disease severity following withdrawal of therapy. [65] In systemic lupus erythematosus patients, negative correlation of CD69 with CD94/NKG2A inactivated γδ TCR bearing T-cell (γδ+ T-cell) reveals that down-regulation of CD94/NKG2A may be due to over-activation of such γδ+ T-cell. [66]
Involvement of Qa-1/HLA-E and CD94/NKG2 system in infectious diseases
It has been proposed that CD94/NKG2 heterodimers may co-stimulate effector functions of differentiated Th1 cells. [67] There are several reports which show CD94/NKG2 expression is markedly up-regulated on CD8 + T-cells during viral and bacterial infections. [68],[69] It has been shown that CD94/NKG2 is capable of hindering the CTL activity against Qa-1 and HLA-E positive cells [43] and recently it has been proposed that it may be involved in attenuation of activation induced cell death, which may possibly help in CD8 + T-cell survival during Listeria monocytogenes infection. [70]
Several reports on the role of MHC-Ib for viral diseases are available. [71],[72],[73] MHC-Ib like HLA-G is found to be over-expressed or up-regulated in immune cells, which is found to be immune suppressive in nature during viral infections. In some viruses like human cytomegalovirus infection, HLA-G is found to be down-regulated by viral US10 protein, unlike classical HLAs. [74] However, nonclassical MHC-I, such as HLA-G is found to be resistant to HIV Nef protein mediated cell surface down-regulation. [75]
Involvement of Qa-1/HLA-E and CD94/NKG2 system in cancer, immune privilege and altered immunity
Association of CD94/NKG2 receptors is found in several cancers, where CD94/NKG2A receptors are found to be widely expressed in tumor infiltrating T-cells. They are found to be involved in blocking tumor lytic activity. [76] In cervical cancer, it has been reported that CD94/NKG2A receptors are up-regulated in tumor infiltrating T-cells compared to normal cervix. This is also found to be correlated with secretion of cytokines like transforming growth factor-beta and interlukin-15 by cervical cancer, which may elevate the CD94/NKG2A receptors. [77] Moreover, it has been shown that Interferon gamma treatment may protect ovarian carcinoma cell lines from CTL lysis through human nonclassical MHC-Is and CD94/NKG2A-dependent mechanism. [78]
In a study with ocular anterior chamber associated immune deviation model in mice, CD94/NKG2 deficient DBA/2J strain of mice have been compared to other mouse strains, where the functional significance of Qa-1-CD94/NKG2A system has been demonstrated in peripheral immune suppression as evident by suppression of antigen-specific delayed-type hypersensitivity (DTH) [Table 3] and [Figure 3]. [79] Moreover, it has been shown that compatibility of Qa-1 haplotype between CD8 + Tregs cells and the immunized recipients is a prerequisite for CD8 + Tregs to suppress the expression of antigen-specific DTH in the recipient mice. [80] The expression of Qa-2, a nonclassical MHC-Ib, has been reported in the corneal endothelium and other substructure lining of the ocular anterior chamber, which suggests that Qa-2 protein may also contribute to the immune-privileged status of the mammalian eye. [81]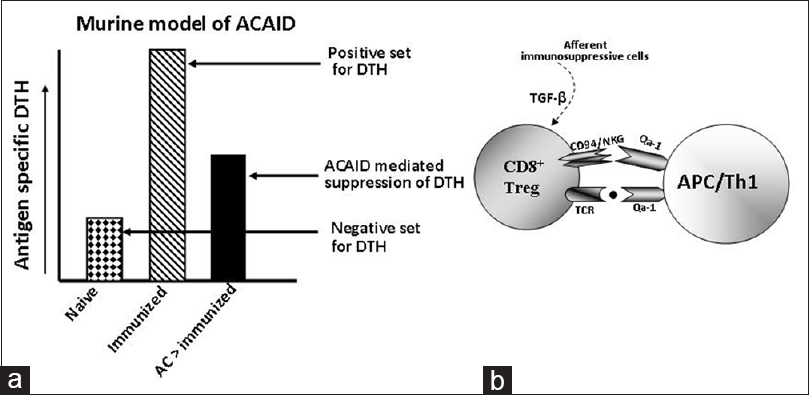 | Figure 3: Qa-1-CD94/NKG2A dependent suppression of delayed type hypersensitivity (DTH) response in anterior chamber associated immune deviation (ACAID) model in mice. ACAID associated suppression of antigen specific DTH is observed in CD94/NKG2A expressing mouse strain, but not in CD94/NKG2A deficient DBA/2J mice. [79] (a) Schematic representation of ACAID model, which illustrate ACAID mediated suppression of antigen mediated DTH in mice. (b) Suggested role of CD94/NKG2A-Qa-1 system for CD8 + immunosuppressive Tregs in ACAID [79] , where transforming growth factor beta may influence the generation of CD8 + Tregs [104],[105],[106],[107]
Click here to view |
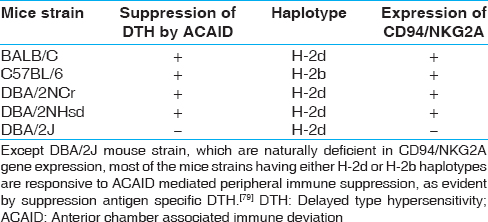 | Table 3: Examples of mouse strains responsive to CD94/NKG2A-Qa-1 associated suppression of antigen specific DTH
Click here to view |
Several groups have demonstrated the involvement of CD94/NKG2 receptors in modulation and regulation of NK cells. [82] However, a study conducted by Vance et al. showed that DBA/2J strain of mice is naturally deficient in CD94/NKG2A receptor expression in adult and neonatal NK cells without disturbing neonatal development. This work suggests that immunological self-tolerance of neonatal NK cells may not be attributed to CD94/NKG2A expression. [83]
Among MHC-Ib molecules, membrane-bound HLA-G and HLA-E have been reported in invasive extravillous trophoblast (EVT) cells and trophoblast cells of decidual tissues, respectively. [84],[85] HLA-G interacts with membrane-bound inhibitory receptors, immunoglobulin-like transcript-2 and -4 (ILT-2 and ILT-4) of monocytes, macrophages, and dendritic cells, respectively. [86],[87] It has also been demonstrated that HLA-G may up-regulate ILT-2, ILT-4 and killer-cell immunoglobulin-like receptor-2DL4 on the membrane of antigen presenting cells, NK cells and CD4 + T-cells without preceding for antigenic co-stimulation. [88] Soluble and membrane-bound HLA-G proteins are found to induce inhibition of T-cell alloproliferation through both ILT-2 and ILT-4. [89] Leukocyte immunoglobulin-like receptor-1 (LIR-1) has been reported to express on surface of a large subpopulation of NK cells, particularly in deciduas and appears to be HLA-G specific, which has immunoregulatory importance during pregnancy. [90] Numerous studies indicate that G*0105N allele frequency increases in recurrent miscarriages and that may function as a risk factor for such loss of pregnancy. [91],[92] However, some reports contradict the role of HLA-G in fetal survival by the detection of G*0105N allele in homozygous adults. [93],[94] Another study suggests that soluble HLA-G (sHLA-G) is present in seminal plasma, and HLA-G is expressed in normal testis and epididymal tissue of male reproductive system. It gives an indication of possible immunoregulatory role of HLA-G in the male reproductive system. [95]
HLA-E is found to regulate CD94/NKG2A receptor-mediated cytolytic activity of NK cells during pregnancy. [85] In another report it has been suggested that HLA-E has a high affinity for NKG2A receptor, which has an inhibitory role than activating NKG2C receptor. [96] Kusumi et al. showed that NKG2A receptors are expressed in most of the decidual CD56 bright NK cells rather than peripheral CD56 dim NK cells. NKG2C expression in CD56 dim is reciprocal to inhibitory NKG2A. In decidual CD56 bright NK cells NKG2A and NKG2C receptors are known to be expressed simultaneously. [97]
In 2003, Ishitani et al. has reported the surface expression of HLA-F in placenta and low expression in syncytiotrophoblast (ST) cells, villous trophoblast (VT) cells and invasive EVT cells. [98] It is contradicting to a study by Nagamatsu et al. where they have found the intracellular expression of HLA-F only in EVT, ST and VT. This variation is probably because they have investigated the placenta from the first stage of gestation, but not of later stages. [99] HLA-F is found to interact with ILT-2 and ILT-4, which expressed on the surface of monocytes and CD19 + B cells, but not on CD56 + NK cells or CD3 + T-cells. [100]
In tumors such as malignant larynx lesions, HLA-G expression is elevated in benign and premalignant lesions and is reduced in invasive carcinomas and in associated draining cervical lymph nodes. However, HLA-E expression was found to be elevated with increased lesion grade, suggesting the expression of HLA-G as an indicator of tumor invasiveness in malignant laryngeal lesions. [101] In ovarian cancer, it is found that the expression of HLA-E plays an important role in neutralizing CTL infiltration. Low expression of HLA-E is found to be associated with enhanced survival rate. [102] Recently, in the mouse B16 melanoma tumor model, it has been showed that activation of CD4 + Foxp3 − T-cells enable melanoma metastasis, which is mediated by Qa-1 dependent suppression of NK-cell cytotoxicity. [103]
 Summary and Future Perspective Summary and Future Perspective | |  |
Here, we have reviewed the gene organization of nonclassical MHC, their phylogenetic analysis and important updates on their interaction with receptors such as TCR, CD94/NKG2 in T, NK, and NKT cells. Moreover, the association of Qa-1/HLA-E to CD94/NKG2 receptor systems with the pathological state of some important diseases and its relation to altered host cell immunity has also been discussed. In brief, the nonclassical MHCs and its receptors CD94/NKG2 are found to be involved in maintaining immune privilege, immune surveillance as a mammalian host protective and beneficial response. However, their effect can be detrimental through an immunosuppressive response during viral infection and cancer/tumor progression. There are many more questions which remain to be explored in future regarding the biology of non-classical MHC-I molecules. Accordingly, specificity of these evolutionary conserved, less-polymorphic, nonclassical MHCs and their receptors towards modulating adaptive immunity is still under investigation. Further studies are warranted to open up new avenues in understanding the nonclassical MHC responses in the perspective of genetic, evolutionary and immunological studies.
 Acknowledgement Acknowledgement | |  |
We are grateful to Professor Robert E. Cone, Department of Immunology, University of Connecticut Health Center, USA for his critical reading and suggestion for the manuscript. [107]
 References References | |  |
| 1. | Bevan MJ. Cross-priming for a secondary cytotoxic response to minor H antigens with H-2 congenic cells which do not cross-react in the cytotoxic assay. J Exp Med 1976;143:1283-8.  [ PUBMED] |
| 2. | Joly E, Rouillon V. The orthology of HLA-E and H2-Qa1 is hidden by their concerted evolution with other MHC class I molecules. Biol Direct 2006;1:2.  |
| 3. | Comiskey M, Goldstein CY, De Fazio SR, Mammolenti M, Newmark JA, Warner CM. Evidence that HLA-G is the functional homolog of mouse Qa-2, the Ped gene product. Hum Immunol 2003;64:999-1004.  |
| 4. | Jones EP, Kumánovics A, Yoshino M, Fischer Lindahl K. Mhc class I and non-class I gene organization in the proximal H2-M region of the mouse. Immunogenetics 1999;49:183-95.  |
| 5. | Lambracht D, Prokop C, Hedrich HJ, Fischer Lindahl K, Wonigeit K. Mapping of H2-M homolog and MOG genes in the rat MHC. Immunogenetics 1995;42:418-21.  |
| 6. | Yoshino M, Xiao H, Jones EP, Fischer Lindahl K. BAC/YAC contigs from the H2-M region of mouse Chr 17 define gene order as Znf173-Tctex5-mog-D17Tu42-M3-M2. Immunogenetics 1998;47:371-80.  |
| 7. | Hashimoto K, Hirai M, Kurosawa Y. Identification of a mouse homolog for the human hereditary haemochromatosis candidate gene. Biochem Biophys Res Commun 1997;230:35-9.  |
| 8. | Volpi EV, Chevret E, Jones T, Vatcheva R, Williamson J, Beck S, et al. Large-scale chromatin organization of the major histocompatibility complex and other regions of human chromosome 6 and its response to interferon in interphase nuclei. J Cell Sci 2000;113:1565-76.  |
| 9. | Hurt P, Walter L, Sudbrak R, Klages S, Müller I, Shiina T, et al. The genomic sequence and comparative analysis of the rat major histocompatibility complex. Genome Res 2004;14:631-9.  |
| 10. | Lader E, Clark BT, Jhanwar SC, Chaganti RS, Bennett D. Definitive chromosomal location of the H-2 complex by in situ hybridization to pachytene chromosomes. Immunogenetics 1985;22:49-54.  [ PUBMED] |
| 11. | Wei XH, Orr HT. Differential expression of HLA-E, HLA-F, and HLA-G transcripts in human tissue. Hum Immunol 1990;29:131-42.  |
| 12. | Braud V, Jones EY, McMichael A. The human major histocompatibility complex class Ib molecule HLA-E binds signal sequence-derived peptides with primary anchor residues at positions 2 and 9. Eur J Immunol 1997;27:1164-9.  |
| 13. | Mittelbronn M, Simon P, Löffler C, Capper D, Bunz B, Harter P, et al. Elevated HLA-E levels in human glioblastomas but not in grade I to III astrocytomas correlate with infiltrating CD8+cells. J Neuroimmunol 2007;189:50-8.  |
| 14. | Derré L, Corvaisier M, Charreau B, Moreau A, Godefroy E, Moreau-Aubry A, et al. Expression and release of HLA-E by melanoma cells and melanocytes: Potential impact on the response of cytotoxic effector cells. J Immunol 2006;177:3100-7.  |
| 15. | Grimsley C, Kawasaki A, Gassner C, Sageshima N, Nose Y, Hatake K, et al. Definitive high resolution typing of HLA-E allelic polymorphisms: Identifying potential errors in existing allele data. Tissue Antigens 2002;60:206-12.  |
| 16. | Ulbrecht M, Couturier A, Martinozzi S, Pla M, Srivastava R, Peterson PA, et al. Cell surface expression of HLA-E: Interaction with human beta2-microglobulin and allelic differences. Eur J Immunol 1999;29:537-47.  |
| 17. | Wolf PR, Cook RG. The TL region gene 37 encodes a Qa-1 antigen. J Exp Med 1990;172:1795-804.  |
| 18. | Hermel E, Hart AJ, Miller R, Aldrich CJ. CTL and sequence analyses of MHC class IB antigens Qa1(c) (H2-T23(r)) and Qa1(d) (H2-T23(f)). Immunogenetics 1999;49:712-7.  |
| 19. | Kastner DL, Rich RR, Shen FW. Qa-1-associated antigens. I. Generation of H-2-nonrestricted cytotoxic T lymphocytes specific for determinants of the Qa-1 region. J Immunol 1979;123:1232-8.  |
| 20. | Aldrich CJ, Rodgers JR, Rich RR. Regulation of Qa-1 expression and determinant modification by an H-2D-linked gene, Qdm. Immunogenetics 1988;28:334-44.  |
| 21. | Aldrich CJ, DeCloux A, Woods AS, Cotter RJ, Soloski MJ, Forman J. Identification of a Tap-dependent leader peptide recognized by alloreactive T cells specific for a class Ib antigen. Cell 1994;79:649-58.  |
| 22. | Kraft JR, Vance RE, Pohl J, Martin AM, Raulet DH, Jensen PE. Analysis of Qa-1(b) peptide binding specificity and the capacity of CD94/NKG2A to discriminate between Qa-1-peptide complexes. J Exp Med 2000;192:613-24.  |
| 23. | Salcedo M, Colucci F, Dyson PJ, Cotterill LA, Lemonnier FA, Kourilsky P, et al. Role of Qa-1(b)-binding receptors in the specificity of developing NK cells. Eur J Immunol 2000;30:1094-101.  |
| 24. | Vance RE, Jamieson AM, Raulet DH. Recognition of the class Ib molecule Qa-1(b) by putative activating receptors CD94/NKG2C and CD94/NKG2E on mouse natural killer cells. J Exp Med 1999;190:1801-12.  |
| 25. | Vance RE, Kraft JR, Altman JD, Jensen PE, Raulet DH. Mouse CD94/NKG2A is a natural killer cell receptor for the nonclassical major histocompatibility complex (MHC) class I molecule Qa-1(b). J Exp Med 1998;188:1841-8.  |
| 26. | Braud VM, Aldemir H, Breart B, Ferlin WG. Expression of CD94-NKG2A inhibitory receptor is restricted to a subset of CD8 + T cells. Trends Immunol 2003;24:162-4.  |
| 27. | Watkins DI, Chen ZW, Hughes AL, Evans MG, Tedder TF, Letvin NL. Evolution of the MHC class I genes of a New World primate from ancestral homologues of human non-classical genes. Nature 1990;346:60-3.  |
| 28. | Watkins DI, Letvin NL, Hughes AL, Tedder TF. Molecular cloning of cDNA that encode MHC class I molecules from a New World primate ( Saguinus oedipus). Natural selection acts at positions that may affect peptide presentation to T cells. J Immunol 1990;144:1136-43.  |
| 29. | Arnaiz-Villena A, Morales P, Gomez-Casado E, Castro MJ, Varela P, Rojo-Amigo R, et al. Evolution of MHC-G in primates: A different kind of molecule for each group of species. J Reprod Immunol 1999;43:111-25.  |
| 30. | Castro MJ, Morales P, Fernández-Soria V, Suarez B, Recio MJ, Alvarez M, et al. Allelic diversity at the primate Mhc-G locus: Exon 3 bears stop codons in all Cercopithecinae sequences. Immunogenetics 1996;43:327-36.  |
| 31. | Boyson JE, Iwanaga KK, Golos TG, Watkins DI. Identification of a novel MHC class I gene, Mamu-AG, expressed in the placenta of a primate with an inactivated G locus. J Immunol 1997;159:3311-21.  |
| 32. | Adams EJ, Parham P. Genomic analysis of common chimpanzee major histocompatibility complex class I genes. Immunogenetics 2001;53:200-8.  |
| 33. | Arnaiz-Villena A, Martinez-Laso J, Alvarez M, Castro MJ, Varela P, Gomez-Casado E, et al. Primate Mhc-E and -G alleles. Immunogenetics 1997;46:251-66.  |
| 34. | Corell A, Morales P, Martínez-Laso J, Martín-Villa J, Varela P, Paz-Artal E, et al. New species-specific alleles at the primate MHC-G locus. Hum Immunol 1994;41:52-5.  |
| 35. | Knapp LA, Cadavid LF, Watkins DI. The MHC-E locus is the most well conserved of all known primate class I histocompatibility genes. J Immunol 1998;160:189-96.  |
| 36. | Lawlor DA, Warren E, Ward FE, Parham P. Comparison of class I MHC alleles in humans and apes. Immunol Rev 1990;113:147-85.  |
| 37. | Otting N, Bontrop RE. Characterization of the rhesus macaque ( Macaca mulatta) equivalent of HLA-F. Immunogenetics 1993;38:141-5.  |
| 38. | Alvarez M, Martinez-Laso J, Varela P, Diaz-Campos N, Gomez-Casado E, Vargas-Alarcon G, et al. High polymorphism of Mhc-E locus in non-human primates: Alleles with identical exon 2 and 3 are found in two different species. Tissue Antigens 1997;49:160-7.  |
| 39. | Boyson JE, McAdam SN, Gallimore A, Golos TG, Liu X, Gotch FM, et al. The MHC E locus in macaques is polymorphic and is conserved between macaques and humans. Immunogenetics 1995;41:59-68.  |
| 40. | Grimsley C, Ober C. Population genetic studies of HLA-E: Evidence for selection. Hum Immunol 1997;52:33-40.  |
| 41. | Urvater JA, Watkins DI. Isolation of the HLA-H orthologue in gorillas and chimpanzees. Immunogenetics 2000;51:69-74.  |
| 42. | Braud VM, Allan DS, O'Callaghan CA, Söderström K, D'Andrea A, Ogg GS, et al. HLA-E binds to natural killer cell receptors CD94/NKG2A, B and C. Nature 1998;391:795-9.  |
| 43. | Lohwasser S, Kubota A, Salcedo M, Lian RH, Takei F. The non-classical MHC class I molecule Qa-1(b) inhibits classical MHC class I-restricted cytotoxicity of cytotoxic T lymphocytes. Int Immunol 2001;13:321-7.  |
| 44. | Houchins JP, Lanier LL, Niemi EC, Phillips JH, Ryan JC. Natural killer cell cytolytic activity is inhibited by NKG2-A and activated by NKG2-C. J Immunol 1997;158:3603-9.  |
| 45. | Lanier LL, Corliss B, Wu J, Phillips JH. Association of DAP12 with activating CD94/NKG2C NK cell receptors. Immunity 1998;8:693-701.  |
| 46. | Ljunggren HG, Kärre K. In search of the 'missing self': MHC molecules and NK cell recognition. Immunol Today 1990;11:237-44.  |
| 47. | Cohen GB, Gandhi RT, Davis DM, Mandelboim O, Chen BK, Strominger JL, et al. The selective downregulation of class I major histocompatibility complex proteins by HIV-1 protects HIV-infected cells from NK cells. Immunity 1999;10:661-71.  |
| 48. | Li J, Goldstein I, Glickman-Nir E, Jiang H, Chess L. Induction of TCR Vbeta-specific CD8+CTLs by TCR Vbeta-derived peptides bound to HLA-E. J Immunol 2001;167:3800-8.  |
| 49. | Pietra G, Romagnani C, Falco M, Vitale M, Castriconi R, Pende D, et al. The analysis of the natural killer-like activity of human cytolytic T lymphocytes revealed HLA-E as a novel target for TCR alpha/beta-mediated recognition. Eur J Immunol 2001;31:3687-93.  |
| 50. | Bendelac A, Rivera MN, Park SH, Roark JH. Mouse CD1-specific NK1 T cells: Development, specificity, and function. Annu Rev Immunol 1997;15:535-62.  |
| 51. | Taniguchi M, Harada M, Kojo S, Nakayama T, Wakao H. The regulatory role of Valpha14 NKT cells in innate and acquired immune response. Annu Rev Immunol 2003;21:483-513.  |
| 52. | Kronenberg M, Gapin L. The unconventional lifestyle of NKT cells. Nat Rev Immunol 2002;2:557-68.  |
| 53. | Arase H, Arase N, Ogasawara K, Good RA, Onoé K. An NK1.1+CD4+8- single-positive thymocyte subpopulation that expresses a highly skewed T-cell antigen receptor V beta family. Proc Natl Acad Sci U S A 1992;89:6506-10.  |
| 54. | Kawano T, Cui J, Koezuka Y, Toura I, Kaneko Y, Motoki K, et al. CD1d-restricted and TCR-mediated activation of valpha14 NKT cells by glycosylceramides. Science 1997;278:1626-9.  |
| 55. | Stanic AK, Shashidharamurthy R, Bezbradica JS, Matsuki N, Yoshimura Y, Miyake S, et al. Another view of T cell antigen recognition: Cooperative engagement of glycolipid antigens by Va14Ja18 natural T (iNKT) cell receptor [corrected]. J Immunol 2003;171:4539-51.  |
| 56. | Miyamoto K, Miyake S, Yamamura T. A synthetic glycolipid prevents autoimmune encephalomyelitis by inducing TH2 bias of natural killer T cells. Nature 2001;413:531-4.  |
| 57. | Ortaldo JR, Young HA, Winkler-Pickett RT, Bere EW Jr, Murphy WJ, Wiltrout RH. Dissociation of NKT stimulation, cytokine induction, and NK activation in vivo by the use of distinct TCR-binding ceramides. J Immunol 2004;172:943-53.  |
| 58. | Parekh VV, Singh AK, Wilson MT, Olivares-Villagómez D, Bezbradica JS, Inazawa H, et al. Quantitative and qualitative differences in the in vivo response of NKT cells to distinct alpha- and beta-anomeric glycolipids. J Immunol 2004;173:3693-706.  |
| 59. | Ota T, Takeda K, Akiba H, Hayakawa Y, Ogasawara K, Ikarashi Y, et al. IFN-gamma-mediated negative feedback regulation of NKT-cell function by CD94/NKG2. Blood 2005;106:184-92.  |
| 60. | Hayakawa Y, Takeda K, Yagita H, Van Kaer L, Saiki I, Okumura K. Differential regulation of Th1 and Th2 functions of NKT cells by CD28 and CD40 costimulatory pathways. J Immunol 2001;166:6012-8.  |
| 61. | Noble A, Zhao ZS, Cantor H. Suppression of immune responses by CD8 cells. II. Qa-1 on activated B cells stimulates CD8 cell suppression of T helper 2 responses. J Immunol 1998;160:566-71.  |
| 62. | Jiang H, Chess L. The specific regulation of immune responses by CD8 + T cells restricted by the MHC class Ib molecule, Qa-1. Annu Rev Immunol 2000;18:185-216.  |
| 63. | Lu L, Kim HJ, Werneck MB, Cantor H. Regulation of CD8 + regulatory T cells: Interruption of the NKG2A-Qa-1 interaction allows robust suppressive activity and resolution of autoimmune disease. Proc Natl Acad Sci U S A 2008;105:19420-5.  |
| 64. | Tang X, Maricic I, Purohit N, Bakamjian B, Reed-Loisel LM, Beeston T, et al. Regulation of immunity by a novel population of Qa-1-restricted CD8alphaalpha+TCRalphabeta+T cells. J Immunol 2006;177:7645-55.  |
| 65. | Walsh CE, Ryan EJ, O'Farrelly C, Golden-Mason L, FitzGerald O, Veale DJ, et al. Differential expression of NK receptors CD94 and NKG2A by T cells in rheumatoid arthritis patients in remission compared to active disease. PLoS One 2011;6:e27182.  |
| 66. | Wang L, Kang N, Zhou J, Guo Y, Zhang X, Cui L, et al. Downregulation of CD94/NKG2A inhibitory receptor on decreased γδ T cells in patients with systemic lupus erythematosus. Scand J Immunol 2012;76:62-9.  |
| 67. | Meyers JH, Ryu A, Monney L, Nguyen K, Greenfield EA, Freeman GJ, et al. Cutting edge: CD94/NKG2 is expressed on Th1 but not Th2 cells and costimulates Th1 effector functions. J Immunol 2002;169:5382-6.  |
| 68. | Moser JM, Gibbs J, Jensen PE, Lukacher AE. CD94-NKG2A receptors regulate antiviral CD8(+) T cell responses. Nat Immunol 2002;3:189-95.  |
| 69. | McMahon CW, Zajac AJ, Jamieson AM, Corral L, Hammer GE, Ahmed R, et al. Viral and bacterial infections induce expression of multiple NK cell receptors in responding CD8(+) T cells. J Immunol 2002;169:1444-52.  |
| 70. | Gunturi A, Berg RE, Forman J. Preferential survival of CD8 T and NK cells expressing high levels of CD94. J Immunol 2003;170:1737-45.  |
| 71. | Huang J, Burke P, Yang Y, Seiss K, Beamon J, Cung T, et al. Soluble HLA-G inhibits myeloid dendritic cell function in HIV-1 infection by interacting with leukocyte immunoglobulin-like receptor B2. J Virol 2010;84:10784-91.  |
| 72. | Chen HX, Chen BG, Shi WW, Zhen R, Xu DP, Lin A, et al. Induction of cell surface human leukocyte antigen-G expression in pandemic H1N1 2009 and seasonal H1N1 influenza virus-infected patients. Hum Immunol 2011;72:159-65.  |
| 73. | Lozano JM, González R, Luque J, Frias M, Rivero A, Peña J. CD8(+) HLA-G(+) regulatory T cells are expanded in HIV-1-infected patients. Viral Immunol 2009;22:463-5.  |
| 74. | Park B, Spooner E, Houser BL, Strominger JL, Ploegh HL. The HCMV membrane glycoprotein US10 selectively targets HLA-G for degradation. J Exp Med 2010;207:2033-41.  |
| 75. | Pizzato N, Derrien M, Lenfant F. The short cytoplasmic tail of HLA-G determines its resistance to HIV-1 Nef-mediated cell surface downregulation. Hum Immunol 2004;65:1389-96.  |
| 76. | Speiser DE, Pittet MJ, Valmori D, Dunbar R, Rimoldi D, Liénard D, et al. In vivo expression of natural killer cell inhibitory receptors by human melanoma-specific cytolytic T lymphocytes. J Exp Med 1999;190:775-82.  |
| 77. | Sheu BC, Chiou SH, Lin HH, Chow SN, Huang SC, Ho HN, et al. Up-regulation of inhibitory natural killer receptors CD94/NKG2A with suppressed intracellular perforin expression of tumor-infiltrating CD8 + T lymphocytes in human cervical carcinoma. Cancer Res 2005;65:2921-9.  |
| 78. | Malmberg KJ, Levitsky V, Norell H, de Matos CT, Carlsten M, Schedvins K, et al. IFN-gamma protects short-term ovarian carcinoma cell lines from CTL lysis via a CD94/NKG2A-dependent mechanism. J Clin Invest 2002;110:1515-23.  |
| 79. | Chattopadhyay S, O'Rourke J, Cone RE. Implication for the CD94/NKG2A-Qa-1 system in the generation and function of ocular-induced splenic CD8 + regulatory T cells. Int Immunol 2008;20:509-16.  |
| 80. | Cone RE, Chattopadhyay S, Sharafieh R, Lemire Y, O'Rourke J. The suppression of hypersensitivity by ocular-induced CD8(+) T cells requires compatibility in the Qa-1 haplotype. Immunol Cell Biol 2009;87:241-8.  |
| 81. | Niederkorn JY, Chiang EY, Ungchusri T, Stroynowski I. Expression of a nonclassical MHC class Ib molecule in the eye. Transplantation 1999;68:1790-9.  |
| 82. | Braud VM, McMichael AJ. Regulation of NK cell functions through interaction of the CD94/NKG2 receptors with the nonclassical class I molecule HLA-E. Curr Top Microbiol Immunol 1999;244:85-95.  |
| 83. | Vance RE, Jamieson AM, Cado D, Raulet DH. Implications of CD94 deficiency and monoallelic NKG2A expression for natural killer cell development and repertoire formation. Proc Natl Acad Sci U S A 2002;99:868-73.  |
| 84. | Ellis SA, Sargent IL, Redman CW, McMichael AJ. Evidence for a novel HLA antigen found on human extravillous trophoblast and a choriocarcinoma cell line. Immunology 1986;59:595-601.  [ PUBMED] |
| 85. | King A, Allan DS, Bowen M, Powis SJ, Joseph S, Verma S, et al. HLA-E is expressed on trophoblast and interacts with CD94/NKG2 receptors on decidual NK cells. Eur J Immunol 2000;30:1623-31.  |
| 86. | Colonna M, Navarro F, Bellón T, Llano M, García P, Samaridis J, et al. A common inhibitory receptor for major histocompatibility complex class I molecules on human lymphoid and myelomonocytic cells. J Exp Med 1997;186:1809-18.  |
| 87. | Colonna M, Samaridis J, Cella M, Angman L, Allen RL, O'Callaghan CA, et al. Human myelomonocytic cells express an inhibitory receptor for classical and nonclassical MHC class I molecules. J Immunol 1998;160:3096-100.  |
| 88. | LeMaoult J, Zafaranloo K, Le Danff C, Carosella ED. HLA-G up-regulates ILT2, ILT3, ILT4, and KIR2DL4 in antigen presenting cells, NK cells, and T cells. FASEB J 2005;19:662-4.  |
| 89. | Naji A, Durrbach A, Carosella ED, Rouas-Freiss N. Soluble HLA-G and HLA-G1 expressing antigen-presenting cells inhibit T-cell alloproliferation through ILT-2/ILT-4/FasL-mediated pathways. Hum Immunol 2007;68:233-9.  |
| 90. | Ponte M, Cantoni C, Biassoni R, Tradori-Cappai A, Bentivoglio G, Vitale C, et al. Inhibitory receptors sensing HLA-G1 molecules in pregnancy: Decidua-associated natural killer cells express LIR-1 and CD94/NKG2A and acquire p49, an HLA-G1-specific receptor. Proc Natl Acad Sci U S A 1999;96:5674-9.  |
| 91. | Aldrich CL, Stephenson MD, Karrison T, Odem RR, Branch DW, Scott JR, et al. HLA-G genotypes and pregnancy outcome in couples with unexplained recurrent miscarriage. Mol Hum Reprod 2001;7:1167-72.  |
| 92. | Pfeiffer KA, Fimmers R, Engels G, van der Ven H, van der Ven K. The HLA-G genotype is potentially associated with idiopathic recurrent spontaneous abortion. Mol Hum Reprod 2001;7:373-8.  |
| 93. | Suárez MB, Morales P, Castro MJ, Fernández V, Varela P, Alvarez M, et al. A new HLA-G allele (HLA-G*0105N) and its distribution in the Spanish population. Immunogenetics 1997;45:464-5.  |
| 94. | Ober C, Aldrich C, Rosinsky B, Robertson A, Walker MA, Willadsen S, et al. HLA-G1 protein expression is not essential for fetal survival. Placenta 1998;19:127-32.  |
| 95. | Larsen MH, Bzorek M, Pass MB, Larsen LG, Nielsen MW, Svendsen SG, et al. Human leukocyte antigen-G in the male reproductive system and in seminal plasma. Mol Hum Reprod 2011;17:727-38.  |
| 96. | Valés-Gómez M, Reyburn HT, Erskine RA, López-Botet M, Strominger JL. Kinetics and peptide dependency of the binding of the inhibitory NK receptor CD94/NKG2-A and the activating receptor CD94/NKG2-C to HLA-E. EMBO J 1999;18:4250-60.  |
| 97. | Kusumi M, Yamashita T, Fujii T, Nagamatsu T, Kozuma S, Taketani Y. Expression patterns of lectin-like natural killer receptors, inhibitory CD94/NKG2A, and activating CD94/NKG2C on decidual CD56bright natural killer cells differ from those on peripheral CD56dim natural killer cells. J Reprod Immunol 2006;70:33-42.  |
| 98. | Ishitani A, Sageshima N, Lee N, Dorofeeva N, Hatake K, Marquardt H, et al. Protein expression and peptide binding suggest unique and interacting functional roles for HLA-E, F, and G in maternal-placental immune recognition. J Immunol 2003;171:1376-84.  |
| 99. | Nagamatsu T, Fujii T, Matsumoto J, Yamashita T, Kozuma S, Taketani Y. Human leukocyte antigen F protein is expressed in the extra-villous trophoblasts but not on the cell surface of them. Am J Reprod Immunol 2006;56:172-7.  |
| 100. | Lepin EJ, Bastin JM, Allan DS, Roncador G, Braud VM, Mason DY, et al. Functional characterization of HLA-F and binding of HLA-F tetramers to ILT2 and ILT4 receptors. Eur J Immunol 2000;30:3552-61.  |
| 101. | Silva TG, Crispim JC, Miranda FA, Hassumi MK, de Mello JM, Simões RT, et al. Expression of the nonclassical HLA-G and HLA-E molecules in laryngeal lesions as biomarkers of tumor invasiveness. Histol Histopathol 2011;26:1487-97.  |
| 102. | Gooden M, Lampen M, Jordanova ES, Leffers N, Trimbos JB, van der Burg SH, et al. HLA-E expression by gynecological cancers restrains tumor-infiltrating CD8? T lymphocytes. Proc Natl Acad Sci U S A 2011;108:10656-61.  |
| 103. | Wang X, Cui Y, Luo G, Wang Q, Hu J, He W, et al. Activated mouse CD4(+) Foxp3(-) T cells facilitate melanoma metastasis via Qa-1-dependent suppression of NK-cell cytotoxicity. Cell Res 2012;22:1696-706.  |
| 104. | Keino H, Masli S, Sasaki S, Streilein JW, Stein-Streilein J. CD8+T regulatory cells use a novel genetic program that includes CD103 to suppress Th1 immunity in eye-derived tolerance. Invest Ophthalmol Vis Sci 2006;47:1533-42.  |
| 105. | Cone RE, Chattopadhyay S, O'Rourke J. Control of delayed-type hypersensitivity by ocular- induced CD8 + regulatory t cells. Chem Immunol Allergy 2008;94:138-49.  |
| 106. | Cone RE, Chattopadhyay S, Sharafieh R, Lemire Y, O'Rourke J, Flavell RA, et al. T cell sensitivity to TGF-beta is required for the effector function but not the generation of splenic CD8 + regulatory T cells induced via the injection of antigen into the anterior chamber. Int Immunol 2009;21:567-74.  |
| 107. | Pais R, Bhowmick S, Chattopadhyay S, Lemire Y, Sharafieh R, Yadav R, et al. An intracameral injection of antigen induces in situ chemokines and cytokines required for the generation of circulating immunoregulatory monocytes. PLoS One 2012;7:e43182.  |
[Figure 1], [Figure 2], [Figure 3]
[Table 1], [Table 2], [Table 3]
|






