|
 
 |
| ORIGINAL ARTICLE |
|
| Year : 2013 | Volume
: 1
| Issue : 1 | Page : 25-29 |
|
The prevalence of HIV sero-positivity in late pregnancy among antenatal attendees with seronegative status in first half of pregnancy in Nnewi, South East Nigeria
Osita s Umeononihu, Joseph I Ikechebelu, John E. N. Okonkwo, Gerald O Udigwe, Ikechukwu I Mbachu
Department of Obstetrics and Gynaecology, Nnamdi Azikiwe University Teaching Hospital, PMB 5025 Nnewi, Anambra, Nigeria
| Date of Acceptance | 23-Dec-2012 |
| Date of Web Publication | 16-Aug-2013 |
Correspondence Address:
Osita s Umeononihu
Department of Obstetrics and Gynaecology, Nnamdi Azikiwe University Teaching Hospital, PMB 5025 Nnewi, Anambra
Nigeria
 Source of Support: None, Conflict of Interest: None  | Check |
 
Background: In sub-Saharan Africa, women and children are vulnerable to HIV/AIDS with about 61% of the infections occurring in women and about 90% of the pediatrics infection through Mother-to-child transmission (MTCT). Antenatal attendees in Nigeria are offered routine HIV testing and counselling on the first visit with opt-out option irrespective of the gestational age at contact. This appears commendable but, considering the national HIV prevalence of 4.6%, our large population of >140 million, the long period of seroconversion of the virus and the fact that pregnant women continue to indulge in activities like: unprotected sexual intercourse with single or multiple partners or men with multiple sexual partners or legal polygamy, cross generational sex, intercourse with sero-discordant partners that put them at risk of new infections; a single screening test on contact may not be sufficient to detect all maternal infections. Aim and Objectives: This is to perform a second HIV testing in antenatal women late in pregnancy and determined the sero-prevalence of HIV amongst those who tested negative in the first half of pregnancy. Materials and Methods: This is a prospective cross sectional study conducted among previously HIV negative pregnant women in Nnamdi Azikiwe University Teaching Hospital (NAUTH) Nnewi between November 2010 and February 2011. The rapid test kits: Determine, Stat Pak and Unigold were used for detection/diagnosis of HIV antibody. Semi structured questionnaire was used to collect socio-demographic data of the subjects. Descriptive analysis of the result was done using the SPSS version 16. Results: The HIV prevalence following repeat testing in late pregnancy was 3.91% (9/230). The mean HIV prevalence at antenatal booking during the study period was 20.64% (116/562). Conclusion: The study highlights the high prevalence of HIV among previously negative attendees in late pregnancy. It brings to the fore the enormity of "missed opportunity" associated with a single routine antibody rapid test for pregnant women on contact/early pregnancy. Therefore, routine repeat antenatal HIV testing and counselling in late pregnancy is strongly advocated. Keywords: Antibody testing, HIV, Nnewi, pregnant women, sero-conversion, sero-prevalence
How to cite this article:
Umeononihu Os, Ikechebelu JI, Okonkwo JE, Udigwe GO, Mbachu II. The prevalence of HIV sero-positivity in late pregnancy among antenatal attendees with seronegative status in first half of pregnancy in Nnewi, South East Nigeria. J HIV Hum Reprod 2013;1:25-9 |
How to cite this URL:
Umeononihu Os, Ikechebelu JI, Okonkwo JE, Udigwe GO, Mbachu II. The prevalence of HIV sero-positivity in late pregnancy among antenatal attendees with seronegative status in first half of pregnancy in Nnewi, South East Nigeria. J HIV Hum Reprod [serial online] 2013 [cited 2017 Apr 6];1:25-9. Available from: http://www.j-hhr.org/text.asp?2013/1/1/25/116533 |
| Introduction | |  |
In 2009, estimated 2.1 million children under 15 years were affected globally with HIV, 370,000 were new infections and 90% of them were in sub-Saharan Africa. [1] Noteworthy is the fact that 90% of these pediatric infections were through MTCT of the virus. [2] The burden of MTCT of HIV is much higher in sub-Saharan Africa due to higher levels of heterosexual transmission, high male: female ratio, high total fertility rate and high levels of breastfeeding. [3] Nigeria has the largest burden in the West African sub region with about 2.98 million people living with HIV. [2] The children epidemic in Nigeria also followed the global trend. Estimated 220,000 children are living with HIV and 90% are from MTCT although a small percentage of "under 15" pediatric infection could be the result of rape or other social abuse.
Despite the relatively low prevalence, Nigeria has the largest HIV disease burden in the sub region, as well as the second highest in the world on account of her large population. [1] This portends negative impact on our economic development due to deterioration in child survival rates, decreasing life expectancy, increasing number of orphans and strain on the weak health systems. [1],[3] If MTCT is not checked, the increasing number of AIDS related deaths in Nigeria may reverse the gains made in child survival.
The virus undergoes a complex interaction with the immune system which results in production of HIV antibodies. The time between infection and antibody development is highly variable and ranges between two weeks to six months with some studies reporting 9 months. [4],[5] This period often termed "the window period" or "period of seroconversion" is usually marked with non-specific symptoms or may be completely asymptomatic but with extremely high viral load, associated high vertical and horizontal transmissibility [6] and poses the greatest challenge to HIV diagnosis in pregnancy using antibody detection techniques. [7] This is because; a minimum quantity of antibody in the serum is required for the test result to be reactive. This can lead to high false negative test result in newly infected pregnant women who will eventually seroconvert in the course of the pregnancy. This will amount to "missed opportunity" [7] if these women are not re-tested later in the pregnancy. Also, new infections can occur in pregnancy after an initial antibody screening test and depending on the gestational age, pass through seroconversion and escape detection due to the current practice of single screening test on contact irrespective of the gestational age. Such women will miss the opportunity to access prevention of mother to child transmission (PMTCT) services which has been proven to reduce MTCT of HIV from as high as 30-45% to less than 2%. [2]
Although the single antibody test algorithm is widely employed and effective in developed countries like the USA where HIV prevalence is low; [8] the same may not be true in a developing country like Nigeria with high HIV prevalence and large population. It may not be out of place therefore to consider the Center for Disease Controls (CDC) recommendation of a double antibody test algorithm (one in early pregnancy and a repeat in late pregnancy) to minimize false negatives and missed opportunities for diagnosis and PMTCT interventions. [7],[8]
This study determined the prevalence of HIV sero-positivity in late pregnancy among antenatal attendees with sero-negative status in first half of pregnancy in Nnewi. This is because, in the natural history of HIV infection, it takes about 2 to 12 weeks for an infected person to develop sufficient HIV antibodies (sero-conversion) for a reactive test result and in some rare cases 6-12 months of sero-conversion period after known exposure have been reported. [9],[10] Thus, sero-conversion is possible during the 40 weeks duration of pregnancy.
| Subjects, Materials and Methods | |  |
This is a prospective cross-sectional study conducted among 230 antenatal attendees in NAUTH Nnewi.
NAUTH is a tertiary health facility and serves as a referral center to Anambra state and its environs. It is a center of excellence for PMTCT of HIV and offers free routine antenatal HIV testing and counselling, as well as treatment, care and support of HIV positive patients.
Inclusion criteria
We included booked pregnant women in late pregnancy that earlier tested negative in the antenatal HIV routine testing and counselling (RTC) and had at least 16 weeks between their initial HIV negative test result and time of the intended second screening.
Exclusion criteria
All pregnant women that withheld consent, pregnant women who are HIV positive from the initial antenatal HIV RTC and known HIV clients were excluded. Furthermore, pregnant women that presented in late pregnancy but whose initial HIV screening was done outside our facility were excluded.
Measure of outcome
The detection of sero-conversion rate in late pregnancy is the primary outcome measure. The demographic predictors of sero-conversion during pregnancy are also of interest.
Methods
The research was conducted after approval from the ethics and research committee of the hospital. The subjects that met the inclusion criteria and gave written or verbal consent were recruited from the antenatal clinics on daily basis Monday through Friday. The consented subjects completed the survey instrument which was a confidential questionnaire.
After completion of the questionnaire, they had pre-test counselling in group while any of the authors or their trained assistants performed a venipuncture and collected about 3 ml of venous blood using disposable syringe and hypodermic needle. The blood was immediately emptied into an EDTA bottle that was coded to match the subject's questionnaire for easy identification. The PMTCT scientists performed a repeat HIV screening according to the national HIV Rapid Testing Serial Algorithm. The folder was tagged to avoid double recruitment of the subject. The result was available in 20-30 minutes and disclosed to the women in an individualized post test counselling session. Most importantly, the positive subjects were enrolled into the PMTCT services.
Statistical analysis
Descriptive analysis of the result was done using Statistical Package for Social Sciences (SPSS) version 16.0.
Limitations of the study
The study did not differentiate between true sero-conversion and new infections in the index pregnancy. Also, this was a hospital based study done in a center of excellence for HIV/PMTCT services. A multi-center study is needed to collaborate or refute these findings.
| Results | |  |
Study background/research information
The study was carried out over four months (November 2010 to February 2011). The total number of antenatal attendees for the period was 638. Seventy-six out of the 638 (11.91%) were previously known HIV positives, 116/562 (20.64%) tested HIV positive in the initial antenatal HIV RTC while 446 (79.36%) tested negative to HIV over the same period [Table 1]. Two hundred and forty five out of the 446 eligible HIV negative attendees were longitudinally recruited into the study. Of these, 15 (6.12%) were lost to follow-up and as such were not included in the analysis. Our analysis was based on the remaining 230 subjects.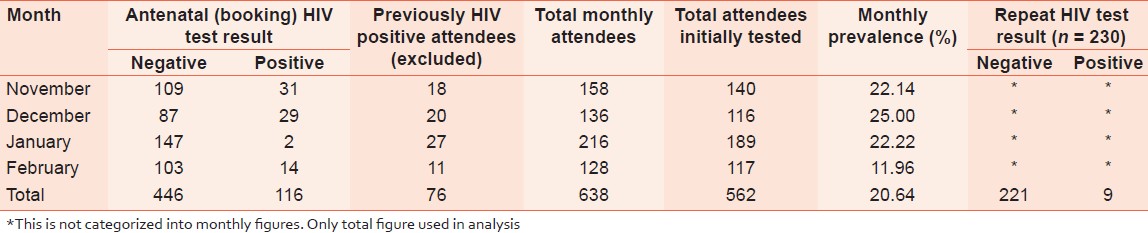 | Table 1: Pattern of HIV status of the antenatal women and repeat HIV status
Click here to view |
Prevalence of repeat HIV serostatus of the pregnant women
Nine out of the 230 women re-screened, tested positive to HIV antibody giving a repeat sero-prevalence of 3.91%. See [Table 1].
Socio-demographic characteristics of the pregnant women
The majority (n = 225; 97.83%) were of the Igbo ethnic group, 4 (1.74%) were Hausas and 1 (0.43%) was from Igede. Majority (n = 228; 99.13%) were Christians while 2 (0.87%) were Muslims. The modal age range was 25-29 years (n = 82; 35.65%) followed by age ≤ 24 years (n = 67; 29.13%) and the least was age ≥ 40 years (n = 4; 1.74%); see [Table 2]. Eight (3.48%) had primary education, 101 (43.91%) had secondary education while the majority (n = 121; 52.61%) had tertiary (post secondary) education. The women were predominantly of middle social class (n = 168; 73.04%) followed by the high social class (n = 35; 15.22%) and low social class (n = 27; 11.74%) respectively.
| Discussion | |  |
Prevalence
This study documents a repeat HIV sero-prevalence of 3.91% among the antenatal attendees who were previously HIV sero-negative earlier in pregnancy. This figure conforms with the CDC's cut off of > (1.2%) for high risk populations. [8],[11] It is higher than the 2.2% seroconversion rate found by Qolohle et al. among antenatal attendees in Durban Southern Africa [12] and 0.4% sero-conversion found among pregnant women with previous HIV sero-negative results in Petersburg Russia. [13] These differences may be due to varying socio-demographic characteristics of the subjects. Many of our subjects were young (modal age: 25-29 years), middle class (73.04%) and upper class (15.22%) women whereas the Durban study was among poor women of low socio-economic class. This result is unexpected but may be due to multiple sexual partners and other confounding variables which were not investigated in our study. Qolohle et al. in their Durban study performed HIV testing using recombinant HIV1/HIV2 Elisa kit for the first antenatal screening and at delivery, our study used the rapid test kits (Determine, Stat-Pak and Unigold) and serial algorithm-2 test method with no further confirmatory test for both the first and repeat screenings. The contribution of the test methods to the differences observed is not yet proven and requires further research. Although the Petersburg study was among women attending high risk maternities, the criterion for high risk was not clearly defined. It is also possible that the associated risks in the study populations are different.
Our study also noted antenatal HIV prevalence rate of 116/562 (20.64%) in four months and monthly prevalence range of 11.96 to 25.00%. This agrees with previous assertions that HIV sero-prevalence shows large variation between and within regions and countries; more so between high and low risk urban populations. [14],[15],[16] The high antenatal prevalence may well be responsible for the high repeat prevalence due to window period. Our finding of nine HIV positive attendees out of 230 previously negative attendees is similar to the 16 out of 342 found by Roongpisuthipong et al. [17] in Bangkok. Unlike in our study, the risk characteristics of the women in the Bangkok study were not stated probably because the study was primarily designed to assess HIV sero-conversion during pregnancy with respect to risk for mother-to-infant transmission.
The high repeat HIV sero-prevalence is unacceptable because it literally means that for every 26 women that screens HIV negative early in the antenatal period, one woman will likely turn positive in late pregnancy. It is difficult to say from the design of this study whether these are new infections or purely true sero-conversion. Nonetheless, the obvious fact is that a single HIV screening in early pregnancy for antenatal attendees in our environment is in-adequate and definitely leads to true "missed opportunity" because these women had availed themselves of antenatal care and testing. We therefore suggest the application of Samson et al. advice that: those health care providers seeing women in communities with HIV incidence of one per 1000 person-years or higher should strongly consider implementing a second routine antenatal HIV testing and counselling during the third trimester. [18]
| Conclusion | |  |
There is high prevalence (3.91%) of HIV sero-positivity among antenatal attendees who were previously sero-negative in early routine antenatal HIV testing and counselling in our center. The factors associated with this sero-conversion could be many and varied. It is truly a "missed opportunity" for these women who had availed themselves of quality care if they are not identified.
Further studies
There is need for larger case controlled studies to determine the factors associated with a repeat positive HIV status in late pregnancy amongst previously negative women. It is also necessary to establish genuine sero-conversion from errors due to test methods in view of studies demonstrating less than the WHO recommended sensitivity and specificity for the rapid test kits.
Institutional and local prevalence studies should be accelerated as it is obvious that the national and state prevalence is at variance with local and institutional prevalence.
| References | |  |
| 1. | UNAIDS Report on the Global AIDS Epidemic. 2010. p. 10. Available from: www.unaids.org/en/media/unaids/contentassets/documents/unaidspublication/2010/20101123_globalreport_en.pdf [Last accessed on 2012 Aug 05]. 
|
| 2. | National Guidelines on Prevention of Mother to Child Transmission of HIV in Nigeria. 4 th ed. FMOH Abuja Nigeria: FMOH; 2010. p. 1-113. 
|
| 3. | Ogaji DS, Ikpeme BM, Oyo-Ita AE, Omuemu VO, Etuk SJ, Ekabua JE. Awareness and acceptability of strategies for preventing mother to child transmission of HIV among antenatal clients in Calabar Nigeria. Niger J Med 2008;17:29-32. 
[PUBMED] |
| 4. | Busch MP, Satten GA. Time course of viremia and antibody seroconversion following human immunodeficiency virus exposure. Am J Med 1997;102:117-24. discussion 125-6. 
|
| 5. | Philippe V, Robert A, Muriel D, Christian C, Dominique P, Jean-Louis T, et al. HIV seroconversion interval and demographic characteristics: No evidence of selection bias. Sex Transm Infect 2001;77:446-8. 
|
| 6. | Olaitan A, Johnson MA. Human immunodeficiency virus in obstetrics. In: Studd J, editor. Progress in Obstetrics and Gynaecology. Vol. 13. Churchill Livingstone Edinburgh; 1998. p. 27-41. 
|
| 7. | Center for Disease Control. Perinatal counselling and guidelines consultation: Revised guidelines for HIV screening of pregnant women. Atlanta Georgia; 1999. 
|
| 8. | Branson BM, Handsfield HH, Lampe MA, Janssen RS, Taylor AW, Lyss SB, et al.; Centers for Disease Control and Prevention (CDC). Revised recommendations for HIV testing of adults, adolescents and pregnant women in health care settings. MMWR Recomm Rep 2006;55(RR-14):1-17. 
|
| 9. | Richard H. Human immunodeficiency virus and AIDS: The course of the disease. Microbiology Immunology online 2009. Available from: http://pathmicro.med.sc.edu/book/welcome.htm [Last accessed on 2012 Aug 05]. 
|
| 10. | Ciesielski CA, Metler RP. Duration of time between exposure and seroconversion in healthcare workers with occupationally acquired infection with human immunodeficiency virus. Am J Med 1997;102:115-6. 
[PUBMED] |
| 11. | Minnesota Department of Health. Clinicians guide to routine HIV testing during pregnancy. USA: Minnesota; 2007. p. 1-6. 
|
| 12. | Qolohle DC, Hoosen AA, Moodley J, Smith AN, Mlisana KP. Serological screening for sexually infections in pregnancy: Is there any value in re-screening for HIV and screening at the time of delivery? Genitourin Med 1995;71:65-7. 
[PUBMED] |
| 13. | Kissin DM, Akatova N, Rakhmanova AG, Vinogradova EN, Voronin EE, Jamieson DJ, et al. Rapid HIV testing and prevention of perinatal HIV transmission in high risk maternity hospital in St Petersburg Russia. AM J Obstet Gynecol 2008;198:183.e1-7. 
|
| 14. | UNAIDS. Joint United Nations Programme on HIV/AIDS: Report on the global AIDS epidemic. Geneva, Switzerland: UNAIDS; 2009. p. 1-100. 
|
| 15. | Nwokedi EE, Azeez-Akande O. The trend of HIV infection in Kano, Nigeria: A seven year study of adult attendees of Aminu Kano teaching hospital. Niger J Med 2007;16:344-7. 
[PUBMED] |
| 16. | Oduwole MD, Salako AA, Gbadamoisi BA, Dawodu O, Alausa OK, Obasanjo-Bello I. Prevalence of HIV infection in Nigeria: Comparison of National sentinel survey and routine data collection in Ogun state Nigeria. 16th Int Conf AIDS 2006; abstract no: MOPE0554. 
|
| 17. | Roongpisuthipong A, Siriwasin W, Simonds RJ, Sangtaweesin V, Vanpraper N, Wasi C, et al. HIV seroconversion during pregnancy and risk for mother-to-infant transmission: Bangkok Collaborative Perinatal HIV Transmission Study Group. J Acquir Immune Defic Syndr 2001;26:348-51. 
|
| 18. | Samson SL, Jamieson DJ, Farnham PG, Bulterys M, Fowler MG. Human immunodeficiency virus retesting in pregnancy: Costs and effectiveness in preventing perinatal transmission. Obstet Gynecol 2003;102:782-90. 
|
[Table 1], [Table 2]
|