|
 
 |
| ORIGINAL ARTICLE |
|
| Year : 2015 | Volume
: 10
| Issue : 1 | Page : 1-7 |
|
Organ toxicity of monosodium glutamate in adult albino Wistar rats
OE Ogbuagu, IN Nweke, PC Unekwe
Department of Pharmacology and Therapeutics, College of Medicine and Health Sciences, Abia State University, Uturu, Abia State, Nigeria
| Date of Web Publication | 4-Nov-2015 |
Correspondence Address:
Dr. O E Ogbuagu
Department of pharmacology and Therapeutics, Faculty of Clinical Medicine, Abia State University Teaching Hospital, P.M.B 7004, Aba, Abia State
Nigeria
 Source of Support: None, Conflict of Interest: None  | Check |
DOI: 10.4103/9783-1230.169056

Objectives: To determine the LD50 and organ toxicity of monosodium glutamate (MSG). Materials and Methods: A total of 30 adult albino Wistar rats of both sexes were used in this study. Fifteen of them were used for acute toxicity test. The second stage of the experiment was performed with 15 rats which received 500 mg/kg, 750 mg/kg, 1000 mg/kg, and 1250 mg/kg of MSG thoroughly mixed with the feeds, respectively, with unlimited supply of drinking water on daily basis for 8 weeks. Group E, which was the control, received an equal amount of feeds without MSG. The animals' weights were measured weekly, constantly observed daily for systemic effects and later sacrificed at the end of 8 weeks in a desiccator under anesthesia with ether following an overnight fast. Blood was obtained by left ventricular cardiac puncture for biochemical analysis. The kidneys, heart, lungs, spleen, liver, and testis were dissected and fixed in 10% formal saline for histological examination using hematoxylin and eosin (Hand E) methods. Results: There was a significant increase in the mean weight of the animals and controls (P < 0.05). There was a significant decrease in the mean serum cholesterol levels, a significant increase in the mean fasting plasma glucose, and plasma alanine transferase levels of the experimental animals when compared with the controls (P < 0.05). The histological finding revealed a reduction in spermatocytes in testes of animals fed with 1000 mg/kg MSG plus necrosis and scanty seminiferous tubules. There was massive necrosis on the liver and lungs, fatty change in the spleen and degenerative changes of heart muscle cells. There were also irregular spaces of various shapes and sizes and necrosis in the kidney. Conclusion: The study demonstrated that consumption of MSG has deleterious effects in virtually all organs which will apparently affect their functions. Keywords: Albino rats, monosodium glutamate, organ, toxicity
How to cite this article:
Ogbuagu O E, Nweke I N, Unekwe P C. Organ toxicity of monosodium glutamate in adult albino Wistar rats. J Med Investig Pract 2015;10:1-7 |
How to cite this URL:
Ogbuagu O E, Nweke I N, Unekwe P C. Organ toxicity of monosodium glutamate in adult albino Wistar rats. J Med Investig Pract [serial online] 2015 [cited 2018 Aug 17];10:1-7. Available from: http://www.jomip.org/text.asp?2015/10/1/1/169056 |
| Introduction | |  |
Noxious substances abound in our environment. We come in contact with them every day, either directly or indirectly. In either way, they may have an effect on our organs in particular and health in general. Few of these noxious substances such as carbon monoxide are inhaled; some such as are alcohol and tobacco are consumed, and much of these substances are added to our food in the form of additives. Most food additives act either as preservatives or enhancers of palatability. One of such food additive is monosodium glutamate (MSG). It is sold in most open markets and stores in Nigeria as "Ajinomoto," marketed by West African Seasoning Company Limited, as "Vedan" or "white magi" marketed by Mac and Mei (Nig) Limited.
The flavor-enhancing power of MSG was discovered to be glumate by Dr. Kikunae Ikeda in 1908.[1] Glutamate is one of the most commonly occurring amino acids. It plays a crucial role in a broad variety of metabolic process, such as in the detoxification of drugs.[2] It is also responsible for 75% of the excitatory neurotransmission in the brain.[3] MSG is readily utilized by glutamate receptors throughout the mammalian body. These receptors could be found in the brain, spinal cord, conducting system; nerve terminals, kidney, liver, testis, lungs, spleen, and heart. Therefore, advocates of food safety should consider these organs as potential sites of damage.[4] Neural injury associated with trauma, stroke, epilepsy, and many neurodegenerative diseases such as Alzheimer's, Huntington's, Parkinson's disease, and Amyotrophic lateral sclerosis was reported to have been mediated by excessive activation of the glutamate receptors.[5] Thus, the free glutamic acid can get into the brain injuring and frequently killing neurons.[6]
Adverse systemic reactions associated with consumption of MSG include: Arrhythmias, hypertension, angina, myalgia, arthritis, depression, dizziness, anxiety, hyperactivity, insomnia, numbness, seizures, diarrhea, nausea and vomiting, shortness of breath, nocturia, oligozoospermia.[7]
Food containing MSG can be identified in the market with the following names: Enzyme modified, anything protein fortified or fermented, autolyzed yeast, broth, bouillon, natural flavoring, gelatin, olyzed protein, pectin, soy protein, sauce, stock, whey protein, yeast extract, and yeast food.[8]
MSG has been used in newborn laboratory mice to induce adult obesity.[9] This is because of the lesion it provokes in the arcuate nucleus of the hypothalamus, resulting in increased caloric intake above utilization of calories.[3] MSG has been shown to trigger epileptic convulsions in rats. The severity of the convulsions and death incidence increased progressively with age.[10]
MSG has a toxic effect on the testis by causing significant oligozoospermia and increased abnormal sperm morphology in a dose-dependent fashion in adult male Wistar rats.[11] It has been implicated in male infertility, by causing testicular hemorrhage, degeneration, and alteration of sperm cell population and morphology.[12]
It has been reported that MSG has neurotoxic effects resulting in brain damage,[13] retinal degeneration, endocrine disorder, and some pathological conditions such as addiction, stroke, epilepsy, brain trauma, neuropathic pain, schizophrenia, anxiety, Parkinson's disease, Alzheimer's disease, Huntington's disease, and amyotrophic lateral sclerosis.[14]
| Materials and Methods | |  |
Thirty adult Wistar rats of both sexes with average weight 160–200g were used for the study. Fifteen rats were used for acute toxicity test and the remaining 15 for the second stage of the experiment.
They were each assigned into five groups: Treatment group A, B, C, and D of n = 12 and control group of n = 3. The animals were obtained and bred at uniform conditions in the animal house of the Department of Pharmacology, College of Medicine, Abia State University, Uturu, Nigeria. They were acclimatized for 7 days before the study. The animals had free access to standard animal feeds purchased from a local commercial supplier and water adlibitum and housed under standard conditions of temperature (25°C ± 20°C), under 12 h light-darkness cycles. The MSG (3 g/satchet containing 99% MSG) was obtained from a grocery store at New Market, Aba in Abia State.
Monosodium glutamate administration
The rats in the treatment group A, B, C, and D were given, respectively, 500 mg/kg, 750 mg/kg, 1000 mg/kg, and 1250 mg/kg of MSG thoroughly mixed with their feeds, respectively, on a daily basis for 8 weeks. The control group E received equal amounts of feeds without MSG. The animals were sacrificed at the end of 8 weeks under anesthesia with ether after an overnight fast. About 3 ml of blood was obtained by left ventricular cardiac puncture from the rat and placed in a container for biochemical analysis. The kidneys, heart, lungs, spleen, liver, and testis were quickly dissected and fixed in 10%, formal saline for routine histological technique.
Histological study
The tissues were dehydrated in ascending grade of alcohol (ethanol), cleared in xylene and embedded in paraffin wax. Serial sections of 6 microns thick were obtained using a rotatory microtome. The deparaffinized sections were stained routinely with hematoxyline and eosin (H and E). Photomicrographs of the desired sections were made for further observations.
Blood collected from the rats was used for fasting plasma glucose, cholesterol, and serum Alanine transaminase (ALT) estimation. Plasma glucose was assayed by Trinder (oxidase-peroxidase system).[15] The serum cholesterol was determined by the method of enzymatic hydrolysis and oxidation.[15] The serum ALT activity was assayed by a method of Reitman and Frankel.[16] All determinations were performed within 24 h of a collection of sample.
Data were analyzed for mean, standard deviation, and compared for significance between the control and experimental groups using Chi-square. The level of significance for all experiment was P < 0.05.
| Results | |  |
The median lethal dose LD50 was found to be 500 mg/kg. This is the median of 200 mg/kg which did not kill any of the animals so administered and 800 mg/kg that killed all its animals.
Two weeks into the study, most of the animals in the experimental group became hyperactive. Four weeks later, one of the animals in the group fed with 1250 mg/kg of MSG developed bulging of eyeballs (exophthalmos), blurred vision, and had several bouts of seizures before its demise six days later. Most of the animals gained maximal weight in the course of the study.
[Table 1] presents the weights of the experimental animals and the control. There was a significant increase in the mean weights of the treated animals when compared to their control, as well as among the different groups (P < 0.05).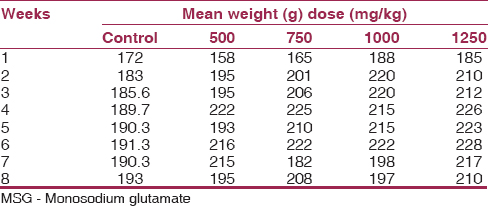 | Table 1: Mean weekly weights of adult Wistar rat treated with different doses of MSG
Click here to view |
[Table 2], [Table 3], [Table 4] show the mean serum cholesterol, glucose, and plasma ALT levels between the experimental and control. There was a significant decrease in the mean serum cholesterol and increase in the mean fasting plasma glucose and ALT levels of experimental animals compared to their controls (P < 0.05).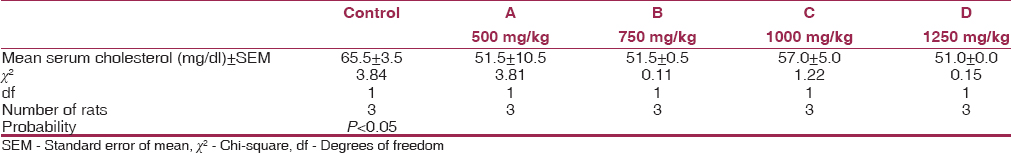 | Table 2: Mean values of serum cholesterol levels for experimental animals and control after 8 weeks
Click here to view |
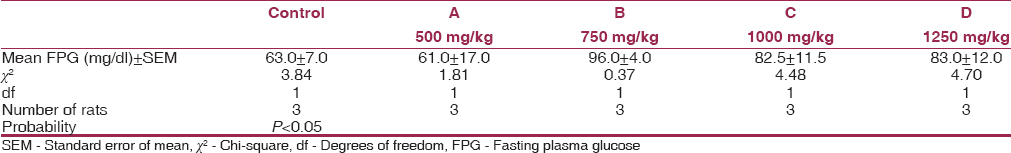 | Table 3: Mean values of FPG for experimental animals and control after 8 weeks
Click here to view |
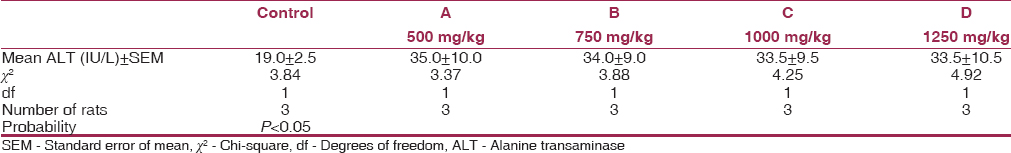 | Table 4: Mean values of plasma ALT levels for experimental animals and control after 8 weeks
Click here to view |
The testes of the animals in the control group showed normal testicular cells with dilatation of ducts [Figure 1]. The treatment section with 1000 mg/kg of MSG after 8 weeks revealed a reduction in spermatocytes, vacuolation, a patchy area of necrosis, and scanty seminiferous tubules [Figure 2].
The rats in the control group showed infiltrates of normal hepatocytes in the liver [Figure 3]. The rats in the treatment group fed with 1000 mg/kg of MSG showed massive necrosis of the liver [Figure 4]. The spleen (control section) showed normal features with lymphocytic infiltrates particularly at the splenic pulp [Figure 5]. Group treated with 1000 mg/kg of MSG showed fatty change [Figure 6].
The control group's hearts had normal cardiac myocytes [Figure 7]. The section treated with 1250 mg/kg of MSG revealed degenerative changes of heart muscle cells and necrosis [Figure 8].
The control group's lungs showed dense mononuclear infiltrates particularly at the alveolar spaces [Figure 9]. The group treated with 1250 mg/kg of MSG revealed necrosis and dissolution of lung tissue due to cell death [Figure 10].
The control section of the kidney showed normal histological features with detailed cortical parenchyma [Figure 11]. The section treated with 1250 mg/kg MSG showed necrosis and irregular spaces of various shapes and sizes [Figure 12].
| Discussion | |  |
Consumption of MSG worldwide with its attendant toxic effects still remains a concern to medical and food scientists.
A significant increase in weight was observed in rats fed with MSG. This was comparable to a similar study carried out to induce obesity in laboratory mice.[9] This is probably because of the effect that it provokes in the arcuate nucleus of the hypothalamus, resulting in increased caloric intake above utilization.[3] MSG also increases appetite and improves the palatability of the poor quality diet.[17] There was a significant decrease in the mean cholesterol compared with the control. This is in contrast with the work of Savastanon et al. where the cholesterol level of the experimental group was higher than control.[18] This could be attributed to the activation of the enzyme, 3-hydroxy-3-methoxyglutamyl-CoA reductase, which catalyzed the rate-limiting step of cholesterol synthesis.
Concerning glucose level, a significant increase was observed in the treated group compared to the controls. This result compares favorably with the work of Negata et al., where MSG was used to induce obesity in type 2 (noninsulin-dependent) diabetes mellitus in mice due to elevated blood glucose.[19]
A significant increase in the serum ALT was observed in the MSG-treated rats compared to the control [Table 4].
This study was necessitated by the observed indiscriminate use of MSG in Nigeria for seasoning and flavor enhancing purposes without taking into consideration the possible adverse effects from its use over time, even at low concentrations. The ALT enzyme is a sensitive marker of liver damage.[20] Therefore, the increase in serum ALT activity by 1%, 10.7%, and 28.1% in rats fed with MSG of 750 mg/kg, 1000 mg/kg, and 1250 mg/kg respectively, may perhaps be an indication of liver damage.
Thus MSG may be hepatotoxic at 750 mg/kg and above, hence should be avoided during the treatment of liver disorders. Since ALT was found to be a positive indicator of insulin resistance, diabetes mellitus and obesity,[21] which are risk factors for coronary disease,[22] the use of MSG at even low dose should not be encouraged because of the possible untoward implications.
The results of the H and E staining of the test is showed reduction in spermatocytes, vacuolations, patchy areas of necrosis and scanty seminiferous tubules of the group treated with 1000 mg/kg of MSG compared to the control. The vacuolations observed may be due to MSG interference.[11]
In the liver, with increasing dose of MSG there was massive necrosis in the central vein and distortion of its cytoarchitecture compared to the control section. This distortion could be associated with functional changes that may be detrimental to health and may affect the hemopoietic function of the liver resulting in cell death. This occurs as a controlled event involving a genetic program in which caspase enzymes are activated.[23]
In the spleens of the treated group, there was generalized fatty change, tissue hypertrophy, and massive infiltration of inflammatory cells. Histological features in the control group were normal. The fatty change reported in this study may have been induced by the toxic level of MSG. This can also be seen in the liver, muscle, and kidney. Other causes of fatty change (steatosis) include protein malnutrition, diabetes mellitus, obesity, and anoxia. In industrialized nations, alcohol abuse is so far the most common cause of fatty change, especially in the liver.[24]
In the heart, there were degenerative changes of the cardiac muscles and necrosis. The mechanism by which MSG does this needs further investigation.
In the kidney, there was necrosis, reduction in the number of renal corpuscles and irregular shapes of various sizes in the treated group which was at variance with the control.
The necrosis observed may have been due to high doses of MSG on the kidney. Pathological or accidental death is regarded as necrotic and could result from extrinsic insults to the cell as osmotic, thermal, toxic, and traumatic effects.[25]
| Conclusion | |  |
Our study demonstrates that consumption of MSG has deleterious effects on virtually all the organs which will apparently affect their functions. Such negative effects call for caution in its use as a flavor enhancer and in industries.
It is, therefore, recommended that further studies be carried out to establish the mechanism by which effects its deleterious effects in the body.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| References | |  |
| 1. | Ikeda K. New seasonings. Chem Senses 2002;27:847-9.  |
| 2. | Meister A. Biochemistry of glutamate. Glutamine and glutathione. In: Filer LJ Jr, Garattini S, Kare MR, Reynolds WA, Wurtman RJ, editors. Glutamic Acid: Advances in Biochemistry and physiology, Raven, New york; 1979. p. 69-84.  |
| 3. | Ganong W. Review of Medical Physiology. 22 nd ed. Singapore: Appleton and Lange, Lange Medical Publication; 2005. p. 107-8, 235.  |
| 4. | Gill S, Mueller RW, McGuire PF, Pulido OM. Potential target sites in peripheral tissues and excitatory neurotransmission and excitotoxicity. Bureau of chemical safety. Health protection branch Canada, Ottawa. Toxicol Pathol 2000;28:26-8.  |
| 5. | Beal MF. Mechanisms of excitotoxicity in neurologic diseases. FASEB J 1992;6:3338-44.  |
| 6. | |
| 7. | |
| 8. | |
| 9. | |
| 10. | Arauz-Contreras J, Feria-Velasco A. Monosodium-L-glutamate-induced convulsions – I. Differences in seizure pattern and duration of effect as a function of age in rats. Gen Pharmacol 1984;15:391-5.  [ PUBMED] |
| 11. | Onakewhor JU, Oforofuo IA, Singh SP. Chronic administration of monosodium glutamate induced oligozoospermia and glycogen accumulation in Wistar rat testes. Afr J Reprod Health 1998;2:190-7.  |
| 12. | Oforofuo IA, Onakewhor JU, Idaewor PE. The effect of chronic administration of MSG on the histology of the adult Wistar rat testes. Biosci Res Commun 2002;9:26-8.  |
| 13. | Eweka AO, Adjene JO. Histological studies of the effects of monosodium glutamate on the medial geniculate body of adult Wistar rat. Electron J Biomed 2007;2:9-13.  |
| 14. | Samuels A. The toxicity/safety of processed free glutamic acid (MSG): A study in suppression of information. Account Res 1999;6:259-310.  [ PUBMED] |
| 15. | Trinder P. Determination of blood glucose using an oxidase-peroxidase system with a non-carcinogenic chromogen. J Clin Pathol 1969;22:158-61.  [ PUBMED] |
| 16. | Reitman S, Frankel S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol 1957;28:56-63.  [ PUBMED] |
| 17. | Colucci PE, Grovum WL. Factors affecting the voluntary intake of food by sheep 6. The effect of monosodium glutamate on the palatability of straw diets by sham-fed and normal animals. Br J Nutr 1993;69:37-47.  |
| 18. | Savastano S, Di Somma C, Belfiore A, Guida B, Orio F Jr, Rota F, et al. Growth hormone status in morbidly obese subjects and correlation with body composition. J Endocrinol Invest 2006;29:536-43.  |
| 19. | Nagata M, Suzuki W, Iizuka S, Tabuchi M, Maruyama H, Takeda S, et al. Type 2 diabetes mellitus in obese mouse model induced by monosodium glutamate. Exp Anim 2006;55:109-15.  |
| 20. | Al-Mamary M, Al-Habori M, Al-Aghbari AM, Baker MM. Investigation into the toxicological effects of Catha edulis leaves: A short term study in animals. Phytother Res 2002;16:127-32.  |
| 21. | Chung RT, Casson DR, Murray G, Song S, Grinspoon S, Hadigan C. Alanine aminotransferase levels predict insulin resistance in HIV lipodystrophy. J Acquir Immune Defic Syndr 2003;34:534-6.  [ PUBMED] |
| 22. | Grundy SM. Hypertriglyceridemia, insulin resistance, and the metabolic syndrome. Am J Cardiol 1999;83:25F-9F.  |
| 23. | Waters CM, Moser W, Walkinshaw G, Mitchell IJ. Death of neurons in the neonatal rodent and primate globus pallidus occurs by a mechanism of apoptosis. Neuroscience 1994;63:881-94.  |
| 24. | Lee RJ, editor. Fatty change and steatohepatitis. In: Diagnostic Liver Pathology. St. Louis: Mosby Year Book; 1994. p. 167.  |
| 25. | Farber JL, Chien KR, Mittnacht S Jr. Myocardial ischemia: The pathogenesis of irreversible cell injury in ischemia. Am J Pathol 1981;102:271-81.  [ PUBMED] |
[Figure 1], [Figure 2], [Figure 3], [Figure 4], [Figure 5], [Figure 6], [Figure 7], [Figure 8], [Figure 9], [Figure 10], [Figure 11], [Figure 12]
[Table 1], [Table 2], [Table 3], [Table 4]
|