|
 
 |
| ORIGINAL ARTICLE |
|
| Year : 2015 | Volume
: 3
| Issue : 2 | Page : 101-107 |
|
Does self-etching primer safely bond bleached teeth? An in-vitro study
Arjun Karra1, Mohammadi Begum Khan2
1 Departments of Orthodontics and Dentofacial Orthopedics, Army College of Dental Sciences, Secunderabad, India
2 Drs. Sudha and Nageswara Rao Pinnamineni Siddhartha Institute of Dental Sciences, Gunnavaram, Vijayawada, Andhra Pradesh, India
| Date of Web Publication | 15-May-2015 |
Correspondence Address:
Mohammadi Begum Khan
Room No.109, Post Graduate Students Girls Hostel, Drs. Sudha and Nageswara Rao Pinnamineni Siddhartha Institute of Dental Sciences Campus, Gunnavaram, Vijayawada - 521 286, Andhara Pradesh
India
 Source of Support: None, Conflict of Interest: None  | Check |
DOI: 10.4103/2321-3825.149047

Aim: The purpose of this in-vitro study was to compare the effect of carbamide peroxide, 16% clinex-gel bleaching on shear bond strength using conventional bonding and self-etching primer bonding (SEP) and to find out how far the changes affect the shear bond strengths at clinically significant level on extracted human tooth enamel. Materials and Methods: Eighty freshly extracted human premolar teeth having moderate to severe dental fluorosis as per Dean's criteria were collected and divided into four groups of 20 each. The four groups are: Group I: Acid etching followed by bonding with Transbond XT (white), Group II: Bleaching, acid etching followed by bonding with Transbond XT (pink), Group III: SEP followed by bonding with Transbond XT (white). Group IV. Bleaching, acid-etching, followed by bonding with Transbond XT (pink). Results: There was a statistically significant difference between the four groups on comparing the mean shear bond strength of Group I, II, III, and IV by one-way analysis of variance test. Summary and Conclusion: Bleaching and bonding with SEP after 30 days storage have comparably similar shear bond strength to the unbleached acid etching group. Keywords: Adhesive remnant index, artificial saliva, carbamide peroxide bleaching, human premolar teeth, instron machine, shear bond strength
How to cite this article:
Karra A, Khan MB. Does self-etching primer safely bond bleached teeth? An in-vitro study
. J Orthod Res 2015;3:101-7 |
| Introduction | |  |
The basis for the adhesion of brackets to enamel has been enamel etching with phosphoric acid as first described by Buonocore [1] in 1955. Newman [2] first applied these techniques to direct bonding of orthodontic attachments to the tooth surface. Bleaching has been one of the most popular patient requested procedures in dentistry, and tends to improve self-image of both the younger and the older population, may involve internal bleaching of non-vital teeth, [3] external bleaching of vital teeth [4] in the office, using concentrated solutions of peroxide-based tooth-whitening materials. Little is known of their biological and physical effects on the shear bond strength of orthodontic adhesives to human tooth enamel. [4],[5],[6] This process might alter the enamel surface structure in a manner similar to acid etching and may alter shear bond strength values. Few studies have reported on bleaching and its effects on orthodontic bonding with self-etching primer (SEP) and conventional bonding. [7],[8],[9],[10] Most studies in restorative dentistry recommend a waiting period anywhere from 1 day to 4 weeks after bleaching to any bonding procedure. [11],[12],[13]
The purpose of this in-vitro study was to compare the effect of carbamide peroxide 16% clinex-gel bleaching on shear bond strength using conventional bonding and SEP bonding and to find out how far the changes affect the shear bond strengths at clinical significant level on extracted human tooth enamel:
- To evaluate the shear bond strength with conventional bonding in nonbleached and bleached teeth.
- To evaluate the shear bond strength with SEP in nonbleached and bleached teeth.
- Comparison of shear bond strength of self-etching with conventional etching in nonbleached and bleached teeth.
| Materials and Methods | |  |
Preparation of Sample Specimens
The selected teeth were mounted using cold cure acrylic resin in uniform sized aluminum blocks of 1.5 × 1.5" dimension. The teeth were mounted in such a way that the buccal surfaces of teeth were parallel to the direction of force application during the process of testing the shear bond force or perpendicular to the central axis of the aluminium block. The apical one-third of the root surface was covered with acrylic to enhance retention of the teeth. The buccal surfaces were cleaned and polished with a rubber cup and slurry followed by rinsing with water spray and drying with compressed air.
Coding for Groups and Teeth
The mounted specimens were divided into two groups of 40 samples each, and these groups were color coded [Figure 1]a and b]. The teeth in each of these groups were numbered on the outer surface of the aluminum block for easy identification and data recording.
Materials Used
Two different types of adhesives (Transbond XT-3M Unitek, USA and SEP-3M Unitek) were used in this study. The bleaching gel used in this study was 16% carbamide peroxide. Eighty premolar brackets (0.022 inch standard edgewise brackets, Gemini series, 3M Unitek) with a base surface area of 9.806 mm 2 were used. Light emitting diode (LED) was used for curing the specimens for 20 s.
Preparation of Artificial Saliva
Artificial saliva was prepared by dissolving the following components in 1 L of sterile double distilled water.
- 0.4 g Nacl
- 1.21 g Kcl
- 0.78 g Na H 2 PO4.2H 2 0
- 0.005 g Na 2 S.9H 2 0
- 1 g urea
Bonding of brackets to the sample teeth
The specimens were divided into four groups based on different methods of tooth preparation for bonding the brackets.
Group I (37% phosphoric acid followed by bonding with Transbond XT adhesive)-control group:
Twenty premolars were bonded with conventional light cure bonding system (Transbond XT). Teeth were etched with 37% phosphoric acid for 15 s. On the prepared enamel surface, a thin coating of the primer followed by placement of Transbond XT adhesive, cured with LED.
Group II (16% carbamide peroxide bleach followed by 37% phosphoric acid etch and bonding with Transbond XT adhesive):
Twenty premolars were bleached with 16% carbamide peroxide bleaching gel according to manufacturer instructions and stored in artificial saliva at 37°C for 30 days and then etched with 37% phosphoric acid, bonded with Transbond XT. Light cured with LED.
Group III (bonding with Transbond plus SEP and Transbond XT adhesive):
Twenty premolars were treated with Transbond-plus SEP for approximately 3 s with the disposable applicator. Then a moisture-free source was used to deliver a gentle burst of air to the enamel. The brackets were bonded with Transbond XT. Cured with LED light source.
Group IV (16% carbamide peroxide bleach followed by etching with Transbond SEP and bonding with Transbond XT adhesive):
Twenty premolars were bleached with 16% carbamide peroxide bleaching gel, stored in artificial saliva at 37° for 30 days and brackets are etched with Transbond plus SEP and bonded with Transbond XT adhesive.16% carbamide peroxide bleaching gel was applied to the enamel surfaces of the embedded teeth in a layer approximately 1-mm thick for 4 h in 1 day. After completion of 10 consecutive daily bleaching procedures, the specimens were thoroughly rinsed with air water syringe for 30 s, air-dried and stored in 250 ml of artificial saliva solution at 37°. Brackets were bonded with Transbond XT adhesive, light cured with LED.
Measurement of Shear Bond Strength
An Instron Universal Testing Machine was used for determining the bond strength in all the four groups [Figure 2]. 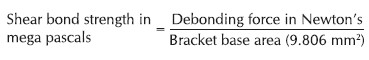
Scanning Electron Microscopy and Adhesive Remnant Index)
All specimens were mounted on carbon stubs and prepared for SEM study by sputtering with gold palladium in a high vacuum evaporator (JFC 1100E Ion sputtering device, JEOL Ltd., Tokyo, Japan) for 6 min.
They were examined in JSM-840A scanning electron microscope (JEOL Ltd., Tokyo, Japan) operated at 20 kV. Photographs were taken at progressively higher magnifications of ×50, ×100, ×500, and ×1000, to view the enamel surface and the adhesive remaining on the enamel surface after debonding [Figure 3] [Figure 4] [Figure 5] [Figure 6].
Adhesive Remnant Index
After failure of the brackets, the amount of adhesive remaining on the tooth surface was measured and adhesive remnant index (ARI) scores were noted using following criteria:
0 = No adhesive remained on the tooth surface.
1 = Less than half of the enamel bonding site was covered with adhesive
2 = More than half of the enamel bonding site was covered with adhesive
3 = The enamel bonding site was entirely covered with adhesive
Method of Statistical Analysis
The following methods of statistical analysis have been used in this study.
1. One-way analysis of variance (ANOVA) was used to test the difference between groups. To find out which of the two groups means is significantly different Post-hoc test of Tukey HSD test is used. Comparison of two variances S a 2 and S b 2 estimated for two group N a and N b subjects, respectively, using F-test.
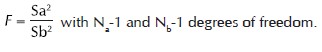
2. The proportion was compared using the Chi-square test of significance. 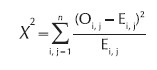
Where O = Observed value and E = Expected value 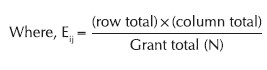
i = row and j = column
n1 and n2 = column total, n1 , n2 , n3 = row total and
n = Grand total,
DF = degree freedom = (row−1) × (column−1)
P < 0.05 was taken to be statistically significant. Statistical Package for Social Science (SPSS, V 16, Transbond XT) was used.
| Results | |  |
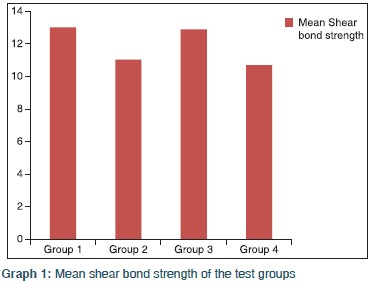
The specimens Groups II and IV were stored in artificial saliva for 30 days at room temperature and then tested for shear bond strength on Instron Universal Testing Machine. The force was recorded in Newton and converted into Mega Pascal. The mean shear bond strength for specimens in Group I was 13.02 ± 1.01 MPa. The corresponding values for Group II, Group III, and Group IV specimens were 11.04 ± 0.97 MPa, 12.89 ± 0.91 MPa, and 10.70 ± 0.68 MPa, respectively [Table 1]. The difference in the mean shear bond strength in different groups was tested with one-way ANOVA. The results revealed statistically significant difference in the mean shear bond strengths between the four groups [Table 2] and Graph 1]. The pair-wise comparison using Post-hoc Tukey HSD test revealed a statistically significant difference between Group I and Group II, Group II and Group III, Group I and Group IV, but no difference between Group I and Group III, Group II and Group IV indicating near to normal shear bond strength following the enamel preparations with a combination bleaching and acid etching, bleaching, and self-etching. This clearly indicated that the adhesive system employed for bonding the brackets did not have any influence on the shear bond strength. | Table 1: Distribution of mean shear bond strength in four different groups
Click here to view |
After the failure of brackets, the ARI score for each sample in all the three groups was noted. The distribution of ARI scores between the four groups was tested using the Chi-square test. The study found a statistically significant difference in the distribution of ARI scores between the four groups [Table 3] and Graph 2]. In Group I, 15% of the samples had an ARI score between 3 and 4 (adhesive mode of bond failure) and 85% of the samples had an ARI scores between 1 and 2 (adhesive-cohesive mode of bond failure). In Group II, 95% of the samples had an ARI score between 4 and 5 (adhesive-cohesive mode of bond failure) and 5% of the samples had an ARI score of 3 (cohesive mode of bond failure). In Group III, 85% of the samples had an ARI score between 1a 2 and 3 (adhesive-cohesive mode of bond failure) and the remaining 15% of the samples had an ARI score between 4 and 5 (cohesive mode of bond failure). None of the samples in Group I had an ARI score of 5 (cohesive mode of bond failure) and none of the samples in Group II and Group IV had an ARI score of 1 (adhesive mode of bond failure). This indicates that the bond failure in Group II and Group IV specimens was adhesive-cohesive or cohesive mode, whereas in Group I, it was adhesive-cohesive or adhesive mode. 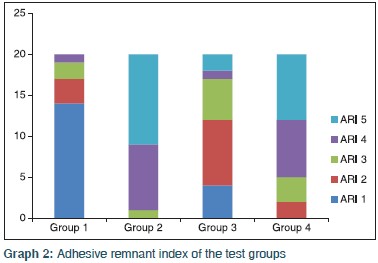
In the present study, there were no statistically significant differences in Group II and Group IV. Group IV showed significantly lower shear bond strength values than the others. The results of the ARI score comparisons [Table 4] in the current study indicated that there were significant differences among the four groups tested. In Groups I and II, there was a higher frequency of ARI scores, indicating cohesive failures within the resin.
| Discussion | |  |
There is concern that vital bleaching could alter the surface topography of enamel and thus affect the bond strength of adhesives to enamel. Carbamide peroxide gel provides 25-35% hydrogen peroxide equivalent and its effect on human enamel composition and topography has been studied by Covington et al. [14] Their results suggested a controlled oxidation process in which the organic phase of the enamel is mobilized without producing grossly unacceptable enamel surface topography. Allison et al. [15] showed an alteration in the topography of acid-etched enamel of carbamide peroxide bleached teeth with the loss of regular prism boundaries when compared with the control. Scherer et al. [16] demonstrated that the use of a brush-on carbamide peroxide gel system for up to 30 days was found to have no effect on surface structure under SEM alterations in bond strength might be significant with regard to clinical operative procedures that involve composite resin bonding, such as bonding orthodontic brackets, porcelain veneers, composite veneers, or future composite restorations. [17] It has been proposed that residual oxygen from the bleaching agent inhibits resin polymerization. [18] Uysal et al. [5] stored their samples in artificial saliva for 30 days and suggested that a bonding delay of a minimum 2-3 weeks might be beneficial.
The following conclusions were made from this study:
- On comparing the mean shear bond strength of Group I, Group II, Group III, and Group IV by one-way ANOVA test, there was no statistically significant difference between the groups (P = 0.000).
- The highest mean shear bond strength on debonding was found in Group I, followed by Group III. Group IV had the lowest mean shear bond strength on debonding.
- The pair-wise comparison between the three groups revealed a statistically significant difference in the mean shear bond strength between Group I and Group II (P = 0.000), Group I and Group III (P = 0.000). There was no statistically significant difference in the mean shear bond strength between Group II and Group IV (P = 0.954).
- There was statistically significant difference in the ARI (Chi-square test) between the three groups (P < 0.05).
- In Group I, 85% of the samples had cohesive mode of bond failure, and 15% of the samples had an adhesive-cohesive mode of bond failure.
- In Group II, 95% of the samples had an adhesive-cohesive mode of bond failure, and 5% of the samples had a cohesive mode of bond failure.
- In Group III, 60% of the samples had a cohesive mode of bond failure, and the remaining 40% of the samples had an adhesive-cohesive mode of bond failure.
- In Group IV, 75% of the samples had an adhesive-cohesive mode of bond failure, and the remaining 25% of the samples had a cohesive mode of bond failure.
| Summary and Conclusion | |  |
It is been mentioned in the literature that many states in India are considered to be endemic fluoride belts. Nalgonda district of Andhra Pradesh is an endemic fluoride belt where the concentration of fluoride in the drinking water ranges from 1.5 to 5 ppm. [19] Though not documented in the available dental literature, the Orthodontists in this area are facing the problem in achieving optimal bond strength and durable bonding. Frequent bracket failure at the compromised enamel interface remains a notable clinical challenge in bonding brackets to fluorosed enamel. The present study was an attempt to check the effect of bleaching and bonding on fluorosed enamel surface with a conclusion that bonding on bleached enamel is safe with SEP.
| References | |  |
| 1. | Buonocore MG. A simple method of increasing the adhesion of acrylic filling materials to enamel surfaces. J Dent Res 1955;34:849-53.  |
| 2. | Newman GV. A post treatment survey of direct bonding of metal brackets. Am J Orthod Dentofacial Orthop 1976;74:197-9.  |
| 3. | Bishara SE, Sulieman AH, Olson M. Effect of enamel bleaching on the bonding strength of orthodontic brackets. Am J Orthod Dentofacial Orthop 1993;104:444-7.  |
| 4. | Uysal T, Basciftci FA, Usümez S, Sari Z, Buyukerkmen A. Can previously bleached teeth be bonded safely? Am J Orthod Dentofacial Orthop 2003;123:628-32.  |
| 5. | Uysal T, Ertas H, Sagsen B, Bulut H, Er O, Ustdal A. Can intra-coronally bleached teeth be bonded safely after antioxidant treatment? Dent Mater J 2010;29:47-52.  |
| 6. | Miles PG, Pontier JP, Bahiraei D, Close J The effect of carbamide peroxide bleach on the tensile bond strength of ceramic brackets: An in vitro study. Am J Orthod Dentofacial Orthop 1994;106:371-5.  |
| 7. | Bulut H, Turkun M, Kaya AD. Effect of an antioxidizing agent on the shear bond strength of brackets bonded to bleached human enamel. Am J Orthod Dentofacial Orthop 2006;129:266-72.  |
| 8. | Gungor AY, Turkkahraman H, Adanir N, Alkis H. Effects of fluorosis and self etching primers on shear bond strengths of orthodontic brackets. Eur J Dent 2009;3:173-7.  |
| 9. | Uysal T, Sisman A. Can previously bleached teeth be bonded safely using self-etching primer systems? Angle Orthod 2008;78:711-5.  |
| 10. | Nour El-din AK, Miller BH, Griggs JA, Wakefield C. Immediate bonding to bleached enamel. Oper Dent 2006;31:106-14.  |
| 11. | Titley KC, Torneck CD, Ruse ND. The effect of carbamide peroxide gel on the shear bond strength of microfilm resin to bovine enamel. J Dent Res 1992;71:20.  |
| 12. | Basting RT, Rodrigues JA, Serra MC, Pimenta LA. Shear bond strength of enamel treated with seven carbamide peroxide bleaching agents. J Esthet Restor Dent 2004;16:250-9.  |
| 13. | Tella S. Evaluation of antimicrobial potential of commonly used denture cleansing methods and denture cleansers. An in vitro study. MAHER Deemed University; 2007.  |
| 14. | Covington JS, Friend GW, Lamoreaux WJ, Perry T. Carbamide peroxide tooth bleaching effects on the enamel composition and topography. J Dent Res 1990;175:530.  |
| 15. | Allison R, Symons AL, Meyers IA. The effect of a vital bleaching technique on the surface integrity of enamel. Aust Dent J 1991;36:312.  |
| 16. | Scherer W, Cooper H, Ziegler B, Vijayaraghavan TV. At-home bleaching system: effects on enamel and cementum. J Esthet Dent 1991;3:54-6.  |
| 17. | Miyazaki M, Sato H, Sato T, Moore BK, Platt JA. Effect of a whitening agent application on enamel bond strength of self-etching primer systems. Am J Dent 2004;17:151-5.  |
| 18. | Josey AL, Meyers IA, Romaniuk K, Symons AL. The effect of a vital bleaching technique on enamel surface morphology and the bonding of composite resin to enamel. J Oral Rehabil 1996;23:244-50.  |
| 19. | Wang WN, Tarng TH. Evaluation of the sealant in orthodontic bonding. Am J Orthod Dentofacial Orthop 1991;100:209-11.  |
[Figure 1], [Figure 2], [Figure 3], [Figure 4], [Figure 5], [Figure 6]
[Table 1], [Table 2], [Table 3], [Table 4]
|