 |
 |

Loperamide-Simethicone vs Loperamide Alone, Simethicone Alone, and Placebo in the Treatment of Acute Diarrhea With Gas-Related Abdominal Discomfort
A Randomized Controlled Trial
Michael A. Kaplan, MD;
Mary Jane Prior, PhD, MPH;
Rita R. Ash, MS;
Kimberly I. McKonly, MS;
Eileen C. Helzner, MD, MS;
Edward B. Nelson, MD, PhD
Arch Fam Med. 1999;8:243-248.
Context Acute diarrhea with gas-related abdominal discomfort is a common, usually self-limited disorder with substantial social and economic impact.
Objective To compare the efficacy and safety of a loperamide hydrochloride–simethicone combination product with those of loperamide alone, simethicone alone, and placebo in treating acute diarrhea with gas-related abdominal discomfort.
Design Randomized, placebo-controlled, double-blind trial of 48 hours' duration.
Setting A primary care, ambulatory practice in Acapulco, Mexico.
Patients A total of 493 outpatient adults aged 18 to 63 years, with acute nonspecific diarrhea with at least moderately severe abdominal discomfort.
Interventions Each patient was randomly assigned to receive 2 chewable tablets containing loperamide hydrochloride, 2 mg, and simethicone, 125 mg (n=124); loperamide hydrochloride, 2 mg (n=123); simethicone, 125 mg (n=123); or placebo (n=123). This was followed by 1 tablet after each unformed stool, up to 4 tablets in any 24-hour period.
Main Outcome Measures Time to last unformed stool and time to complete relief of gas-related abdominal discomfort were the protocol-specified primary outcomes. Secondary outcomes included time to complete relief of diarrhea, number of unformed stools, and patient-assessed variables at the end of the study (overall illness relief, diarrhea relief, and abdominal discomfort relief).
Results Patients who received loperamide-simethicone had significantly (P<.001) shorter time to last unformed stool and faster relief of gas-related abdominal discomfort than patients who received loperamide, simethicone, or placebo alone. Loperamide-simethicone was significantly (P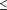 .01) more effective than the other 3 treatments for all end-of-study patient-assessed outcomes and all clinically important secondary outcomes. No significant differences in adverse events were found among treatment groups. .01) more effective than the other 3 treatments for all end-of-study patient-assessed outcomes and all clinically important secondary outcomes. No significant differences in adverse events were found among treatment groups.
Conclusions The loperamide-simethicone combination chewable product provides faster and more complete relief of acute nonspecific diarrhea and associated gas-related abdominal discomfort (gas pain, cramps, gas pressure, and bloating) than either of its components or placebo. The combination is well tolerated.
From the Medical Department, McNeil Consumer Healthcare, Fort Washington, Pa (Drs Kaplan, Prior, Helzner, and Nelson, and Mss Ash and McKonly). Ms Ash is now with Clinical Trials Management, Janssen Research Foundation, Titusville, NJ; Dr Helzner is now with Worldwide Clinical Development and Outcomes Research, Johnson & Johnson Professional Group, Titusville.
RELATED ARTICLE
The Archives of Family Medicine Continuing Medical Education Program
Arch Fam Med. 1999;8(3):207-209.
FULL TEXT
|