 |
 |

The Cost of Antibiotics in Treating Upper Respiratory Tract Infections in a Medicaid Population
Arch G. Mainous III, PhD;
William J. Hueston, MD
Arch Fam Med. 1998;7:45-49.
ABSTRACT
Objective To examine the use and cost of the nonindicated treatment regimens of antibiotics for nonspecific upper respiratory tract infections (URIs) in a Medicaid population.
Design A cross-sectional sample of Kentucky Medicaid claims for 50000 people (July 1, 1993-June 30, 1994).
Setting Episodes of care were created linking outpatient and emergency department visits for URIs to medications filled within a 5-day period.
Participants Individuals who were seen in ambulatory care for a URI as defined by the International Classification of Diseases, Ninth Revision, Clinical Modification codes 460 and 465. Of the 15706 episodes, 95% were outpatient office episodes. The outpatient episodes were accounted for by 8784 patients and 946 physicians.
Main Outcome Measures Use of antibiotics in URI episodes. Proportionate costs and costs per episode were computed based on claims paid by Medicaid.
Results Sixty percent of outpatient episodes and 48% of emergency department episodes resulted in an antibiotic prescription being filled. In outpatient settings, episodes in which secondary diagnoses of either otitis media or acute sinusitis were found accounted for less than 6% of the episodes that resulted in an antibiotic prescription being filled. The most frequently filled antibiotic was amoxicillin, although second- and third-generation cephalosporins were the second most frequently occurring antibiotic class. Twenty-three percent and 9% of outpatient and emergency department episodes, respectively, resulted in a prescription filled for antihistamines. In outpatient episodes, antibiotics account for 23% of the total cost of care. In emergency department visits, antibiotics account for 8% of the cost of URIs. Antibiotics cost, on average, $9.91 for each episode of care in outpatient office visits. An estimate of the cost of antibiotics for URIs in a year for the Kentucky Medicaid program is $1.62 million.
Conclusions The results indicate that a substantial proportion of resources in Medicaid are being used for nonindicated and ineffective treatments for URIs. With the increase in antibiotic-resistant pathogens and shrinking public health care funding, the current treatment for URIs should be reexamined.
INTRODUCTION
UPPER RESPIRATORY tract infections (URIs) are common acute infections and are 1 of the 5 most common reasons for physician office visits.1 Although URIs are mild, self-limited, and of short duration, they are a leading cause of acute morbidity and industrial and school absenteeism.2-4 Despite extensive research efforts, few successful treatments have been identified for URIs.5
Because of the viral cause of URIs, antibiotics are not an indicated treatment. Yet physicians continue to prescribe these drugs for apparently viral URIs. A recent study in the United States showed that 60% of cases of acute nasopharyngitis (common cold) were treated with antibiotics.6 This practice may be extensive in England and Scotland as well. Although they did not specifically link antibiotic prescriptions to URIs, the British Society for Antimicrobial Chemotherapy showed a 45.8% increase in antibioticprescriptions in the community in England and Scotland between 1980 and 1991.7 The authors hypothesized that the increase in antibiotic prescriptions is due to the use of antibiotics for respiratory symptoms.
Antibiotic resistance is a growing problem, with various pathogens demonstrating resistance as a consequence of the extensive use and abuse of antibiotics.8-10 Consequently, the widespread use of antibiotics in viral infections might play a notable role in the development of drug-resistant bacteria.11
Publicly funded health care systems have to deal with shrinking resources and the concomitant decisions about the allocation of finite resources. As financial stress increases for these health systems, they are searching for ways to improve quality and decrease costs. One strategy being used by Medicaid is to move from a fee-for-service system into a managed care system.12 Another strategy is to focus on quality improvement and the cost-effectiveness of the care provided in the program. Thus, the use of ineffective treatments for low-cost, high-volume conditions, such as antibiotics for URIs, has notable implications for the cost of health care as well as the decisions about resource allocation in a publicly funded system with finite resources.
This study examines the cost of antibiotics and other treatment regimens for URIs in a Medicaid population.
PARTICIPANTS AND METHODS
From the 527000 active recipients in the Kentucky Medicaid Program, a random sample of 50000 patients who had at least 1 claim to a physician, dentist, or optometrist between July 1, 1993, and June 30, 1994, was selected from the Kentucky Medicaid claims data. Medicaid is a joint federal and state program that covers a wide range of health-related services and serves people of limited financial resources who have diverse health care needs. All persons in nursing homes and mental health institutions were excluded.
Upper respiratory tract infections were defined by the International Classification of Diseases, Ninth Revision, Clinical Modification codes under the diagnostic stem code of 465, "acute upper respiratory tract infections of multiple or unspecified sites," and code 460, "acute nasopharyngitis/common cold."13 All codes under the stem code of 465 (465.0, 465.8, and 465.9) were used to define URIs because variation may exist between physicians in the codes used for this condition.13 Also defined as a generalized URI was code 460 because the symptom complex designated by the lay term "common cold" represents generalized URIs.2 The selection of various codes to define URIs was based on the awareness that different offices include different precoded International Classification of Diseases, Ninth Revision, Clinical Modification codes on their billing sheets for URIs. All encounters for URIs seen in either an outpatient setting or an emergency department (ED) were available for analysis.
We defined ambulatory care to include outpatient and ED encounters. Many individuals of lower socioeconomic status use EDs as a major source of ambulatory care.14 Patient residence was classified as urban if the individual lived in a county in a metropolitan statistical area and rural if the county of residence was outside a metropolitan statistical area.
With the use of the Medicaid claims data, specific episodes of URIs were identified. Individuals could have more than 1 episode of care in the data set. Each episode begins with a physician claim for outpatient or ED evaluation and management. The evaluation and management Current Procedural Terminology codes indicating physician evaluation and management in an outpatient setting are 99201-5 and 99211-5 and ED codes 99281-5 and 99288.15 All claims on the date of service with the diagnosis of URI are considered to be part of the encounter. Because Kentucky Medicaid drug claims do not have a corresponding diagnosis code, the medications in the URI encounter had to be linked according to the date of service.
The pharmaceutical treatment was assumed to have been prescribed for treatment of the URI if the date of the drug claim was on the day of the physician visit or up to and including 4 days after the physician visit. This period surrounding the physician visit should account for the possible lag between seeing the physician and filling the prescription. Encounters were excluded if any additional visit (either inpatient or outpatient) for another condition occurred some time within the 4 days after the visit for the URI. This was done to provide a window of drug acquisition uncontaminated by other conditions. Further, if another visit for a URI was reported within the same period, the second visit was eliminated from the data set because this visit was presumed to be part of an initial URI episode. In an effort to make a link between the prescribed drug and the encounter for the acute illness, all drugs filled with longer than a 29-day supply were excluded because a 30-day supply should be indicative of medication for a chronic condition. To be as inclusive as possible for all medications prescribed for URIs, yet to limit the inclusion of medications that would not be expected to be prescribed for URIs, the analysis included drugs in the following therapeutic classes: antibiotics, antihistamines, and symptomatic medications (analgesics, bronchodilators, and inhaled corticosteroids). Combination antihistamine and decongestant medications were classified as symptomatic medications because of the effect of the decongestant. Topical or ophthalmic medications were excluded, as were antifungal antibiotics, anthelmintics, and antiprotozoan agents.
Upper respiratory tract infections may have a comorbid condition, such as acute sinusitis or otitis media, that would suggest treatment with antibiotics. Consequently, we examined the secondary diagnoses of acute sinusitis (all codes under the stem code of 461) and otitis media (all codes under the stem codes of 381 and 382) to estimate whether antibiotic use was directed toward a diagnosis other than the URI. The cost estimates were based on claims paid by Medicaid.
RESULTS
Table 1 indicates the sociodemographic characteristics of the episodes of care. Of the 15706 episodes, 95% were outpatient office episodes. The age distribution of the patients in the investigated episodes is skewed, with 48% of the outpatient office episodes being for patients younger than 4 years. Similarly, 61% of the ED episodes were for patients younger than 4 years. Although 72% of the outpatient office episodes were in rural areas, only 51% of the ED episodes were in rural areas.
|
|

|
|
Table 1. Description of Episodes of Care for Upper Respiratory Tract Infections*
|
|
|
Data regarding the use of antibiotics and antihistamines as treatment for URIs in ambulatory episodes of care are as follows (all data are given as the number [percentage] for each type of episode of care):
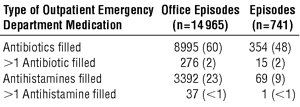
A notable proportion of visits include filled prescriptions for either antibiotics or antihistamines. In several cases, patients received more than 1 antibiotic in an episode of care for a URI. In outpatient office episodes that resulted in an antibiotic prescription being filled, 91% (8149/8995) of the patients filled the antibiotic prescription on the same day as the visit. For ED episodes, 58% (206/354) of the patients filled the antibiotic prescription on the same day as the visit.
In outpatient office episodes, 4% (669/14965) of the patients had a secondary diagnosis of otitis media and less than 1% (57/14965) had a secondary diagnosis of acute sinusitis. Sixty-nine percent (463/669) of the patients with a secondary diagnosis of otitis media and 77% (44/57) of those with a secondary diagnosis of acute sinusitis filled an antibiotic prescription. These episodes with secondary diagnoses that dictated a prescription for antibiotics accounted for 6% of all episodes for which antibiotics were prescribed. In ED episodes, 8% (56/741) of the patients had a secondary diagnosis of otitis media; neither of the 2 patients with a secondary diagnosis of acute sinusitis filled an antibiotic prescription. Among patients with a secondary diagnosis of otitis media, 70% (39/56) filled a prescription for an antibiotic, while no patient with a secondary diagnosis of acute sinusitis filled such a prescription.
Table 2 shows that amoxicillin and other forms of penicillin were the most frequently prescribed antibiotics for URIs in outpatient office episodes and ED episodes. Second- and third-generation cephalosporins were the second most frequently prescribed class of antibiotic.
|
|

|
|
Table 2. Types of Antibiotics Filled for Upper Respiratory Tract Infections*
|
|
|
Six different antihistamines were prescribed in outpatient office episodes, with diphenhydramine hydrochloride accounting for 84% (2878/3429) of the filled prescriptions. Promethazine hydrochloride (247/3429), cyproheptadine hydrochloride (169/3429), and loratadine (130/3429) accounted for 7%, 5%, and 4% of the prescriptions, respectively. Terfenadine and clemastine fumarate accounted for less than 1% of the antihistamines prescribed. In ED episodes of care, only diphenhydramine (59 of 70) and promethazine (11 of 70) were filled antihistamines for URIs.
Various symptomatic medications were filled in outpatient office episodes. Analgesics were the most common medication, accounting for 68% (705/1031) of the prescriptions. Ibuprofen accounted for 41% (287/700) of those prescriptions. Twenty percent (211/1031) of the symptomatic medications was accounted for by the antihistamine and decongestant combination of terfenadine and pseudoephedrine hydrochloride. In ED episodes, analgesics accounted for 75% (21/28) of the prescriptions, while terfenadine-pseudoephedrine accounted for 21% (6/28) of the prescriptions.
Table 3 shows the proportionate cost of antibiotics in episodes of care. In outpatient office visits, 23% of the total cost is for the nonindicated treatment regimen of antibiotics. On average, antibiotics cost $9.91 per episode of care. In ED episodes, this proportion is notably less; however, the additional charges and procedures that accompany ED visits are notably higher than those seen in outpatient office visits.
|
|

|
|
Table 3. Cost of Upper Respiratory Tract Infections
|
|
|
In the outpatient episodes that resulted in an antibiotic prescription being filled, antibiotics accounted for 28% of the total cost, or $18.10 per episode for the inappropriate prescribing of antibiotics. In the ED episodes that resulted in an antibiotic prescription being filled, antibiotics accounted for 13% of the total cost, or $15.34 per episode for the inappropriate prescribing of antibiotics. An estimate of the cost of antibiotics for URIs in the Kentucky Medicaid program, assuming 527000 active cases, would be $1618575.
COMMENT
As publicly funded health systems like Medicaid face economic restructuring and greater scrutiny on the cost and quality of care provided, the examination of low-cost, high-volume services like the treatment of URIs will become particularly important. Our study suggests that most outpatient office episodes of care for URIs have antibiotics as a treatment regimen. The results are similar to a national self-report survey that showed a high rate of antibiotic prescribing for URIs in ambulatory care.16 Antibiotics are not indicated as treatment regimens for URIs; in the case of outpatient office episodes of care, they account for almost one quarter of the cost of providing care for URIs. In Kentucky's Medicaid program, this practice adds an additional $9.91 to each episode of care in outpatient office visits.
The data show that some individuals receive a second antibiotic prescription within the 5-day period. The use of a second antibiotic may indicate the patient's inability to tolerate the first. This finding suggests that prescribing antibiotics is not benign and should be thoughtfully considered. Moreover, the prescription of a second antibiotic continues to increase the cost of treatment of URIs, even though neither the first nor the second antibiotic were indicated.
Additional cost implications follow from the substantial rate of prescribing antihistamines for URIs. Antihistamines, with a few exceptions, have not been shown to be effective in the symptomatic treatment of URIs.5 Diphenhydramine, which accounted for 84% of the outpatient antihistamine prescriptions, has not been shown to be effective for the treatment of URIs.17 Essentially, only chlorpheniramine maleate has shown a benefit in treating URIs, but it was not found to be prescribed in this study.18
Some of the cost in Medicaid pharmaceuticals is for medication that is easily obtained in over-the-counter forms. Specifically, most symptomatic treatment regimens were for ibuprofen or terfenadine-pseudoephedrine. Ibuprofen and pseudoephedrine are available at reasonable costs over-the-counter but are prescribed in Medicaid so that the medication is free to the patient.
A substantial proportion of resources in the management of URIs is being allocated to unnecessary and useless treatments. This finding is particularly important in a publicly funded health system like Medicaid because of the competing priorities for money. For instance, some evidence has shown that childhood immunization distribution programs in Medicaid increase the likelihood of immunization coverage,19 particularly in rural areas.20 This example suggests policy debates regarding the redistribution of money spent on antibiotics and antihistamines for URIs into more effective programs.
These results may be an underestimate of the cost of nonindicated medications for URIs because physicians may code nonspecific URIs as infections that would suggest bacterial origins (eg, acute sinusitis).21 Patients expect to receive a medication prescription at the end of a physician visit.22 This expectation and the belief by practitioners that patients will be dissatisfied with their care and change physicians may play a part in the high rate of antibiotic prescriptions. Physicians may code a condition that is likely to be of viral cause with an alternative diagnosis so that they can satisfy the perceived patient expectations for antibiotics.23
The practice of prescribing antibiotics for URIs has notable implications beyond the fact that the antibiotics are ineffective and unindicated treatments. Evidence exists to indicate that the prevalence of drug-resistant bacteria is increasing. Penicillin-resistant Streptococcus pneumoniae was discovered in more than 50% of the children tested at a child day care in rural Kentucky.24 Similarly, 53% of the children at a child day care center in Nebraska in whom S pneumoniae colonized had isolates highly resistant to penicillin.25 The overuse of antibiotics contributes to emerging infections and the development of drug-resistant bacteria.11
The results suggest a need for a change in physician prescribing behavior. Various interventions, including continuing medical education, practice guidelines, and use profiling, have been used with varying success. First, a guideline on appropriate prescribing for URIs may have use. Unfortunately, adherence to many clinical practice guidelines has been less than optimal. Guidelines with the highest likelihood of being effective are those that are created on a local level with a specific educational intervention.26
Literature on changing physician prescribing behavior suggests that passive educational interventions are generally ineffective.27-29 The process of targeting individual physicians for face-to-face education (known as academic detailing) has been shown to be remarkably effective, particularly in changing antibiotic prescribing behavior.28, 30 Academic detailing can specifically target the physicians who most need to change their practices. However, a major drawback to academic detailing is that it is extremely labor intensive and expensive. A method that has shown particular use in influencing physician behavior and that may have use in changing prescribing behavior for URIs is the profiling of physician practice patterns and the provision of feedback about the physician's performance.31 Profiles are useful in providing information on medical practice because (1) they focus on patterns of practice, rather than instances of care; (2) by using administrative and claims databases, they are efficient and unobtrusive; and (3) they provide information on a practice pattern within the context of a group or a norm. Feedback about an individual's performance can focus on a comparison with one's peers or on a clear norm or standard of performance.32
A possible direction for future research might be to examine the use of practice profiling and feedback as an intervention to change antibiotic prescribing behavior for URIs. In addition, although Medicaid programs vary in the physician characteristics that they collect (Kentucky does not require collection of physician specialty), determining the characteristics of physicians for whom interventions are necessary may be a useful direction for investigation.
This study has several limitations. First, although the data should represent treatment regimens in one state (946 different physicians were included in the outpatient episode analysis), the practices are still based on claims filed to one state's Medicaid system. Practices may vary from state to state. No objective validation of the diagnoses could be made from the available data. Symptoms of nonspecific URIs may overlap with URIs localized to a particular anatomical region (eg, sinusitis), thereby possibly leading to some diagnosis misclassification, particularly with preprinted billing forms. Although the diagnosis could not be objectively evaluated, treatment decisions were made based on the physician's belief in what the physician thought was wrong. The most accurate data available about the physician's impression is the diagnosis code submitted to Medicaid. Further, the physicians might have performed tests for which they did not file a claim. However, because reimbursement for services is dependent on filing claims, the physician has a direct monetary incentive for filing claims for performed services.
Second, because the drug claims contained no diagnosis code, we had to make assumptions about the drug behavior by choosing appropriate medications prescribed in a specified period. Other medications may have been prescribed and not filled or provided as a sample from the physician's office. In addition, we assumed that the medications filled within 4 days of the visit were related to the condition for which the physician billed and were not prescribed at an earlier date or for an unrelated condition that was diagnosed but not submitted as a diagnosis to Medicaid as the reason for the visit. However, in more than 90% of the episodes that resulted in an antibiotic prescription being filled, the antibiotics were received on the same day as the physician visit, suggesting a link between the visit and the prescription.
Third, the filled antibiotic prescription could be a treatment regimen not for the URI but for a secondary diagnosis that would suggest a bacterial cause. At least for coded secondary diagnoses, this does not seem to fully explain the high rate of antibiotic prescribing. Patients receiving antibiotics who had a secondary diagnosis of either acute sinusitis or otitis media account for 6% of the total outpatient office visits that resulted in an antibiotic prescription being filled.
Although some effective treatment regimens have been identified for URIs, ineffective treatment regimens (antibiotics and antihistamines) are commonly used and are a substantial unjustified cost in the treatment of URIs for those in the Medicaid program. Medicaid, as well as private health systems and physicians, should reexamine its treatment program for URIs. Even though medical treatments and technology have advanced in the last century, for the treatment of URIs, Sir William Osler's words still ring true: "There is only one way to treat a cold, ie, with contempt."33
AUTHOR INFORMATION
Accepted for publication December 19, 1996.
Corresponding author: Arch G. Mainous III, PhD, Department of Family Practice, Kentucky Clinic, University of Kentucky, Lexington, KY 40536-0284.
From the Department of Family Practice, University of Kentucky, Lexington (Dr Mainous); and the Department of Family Medicine, University of Wisconsin–Madison, Madison, and Eau Claire Family Practice Residency, Eau Claire, Wis (Dr Hueston).
REFERENCES
1. Schappert SM. National Ambulatory Medical Care Survey, 1991 summary. Vital Health Stat 13. 1994;No. 116.
2. Gwaltney J. The common cold. In: Mandell G, Douglas R, Bennett J, eds. Principles and Practice of Infectious Disease. New York, NY: Churchill Livingstone Inc; 1990:489-493.
3. Koster F. Respiratory infections. In: Barker LR, Burton JR, Zieve PD, eds. Principles of Ambulatory Medicine. Baltimore, Md: Williams & Wilkins; 1987:347-355.
4. Benson V, Marano MA. Current estimates from the National Health Interview Survey. Vital Health Stat 10. 1994;No. 189.
5. Smith MBH, Feldman W. Over-the-counter cold medications: a critical review of clinical trials between 1950 and 1991. JAMA. 1993;269:2258-2263.
FREE FULL TEXT
6. Mainous III AG, Hueston WJ, Clark JR. Antibiotics and upper respiratory infection. J Fam Pract. 1996;42:357-361.
WEB OF SCIENCE
| PUBMED
7. Davey PG, Bax RP, Newey J, et al. Growth in the use of antibiotics in the community in England and Scotland in 1980-93. BMJ. 1996;312:613.
FREE FULL TEXT
8. Burns JL. Mechanisms of bacterial resistance. Pediatr Clin North Am. 1995;42:497-507.
WEB OF SCIENCE
| PUBMED
9. Ballow CH, Schentag JJ. Trends in antibiotic utilization and bacterial resistance. Diagn Microbiol Infect Dis. 1992;15(suppl 2):37S-42S.
10. Bartlett JG, Froggatt III JW. Antibiotic resistance. Arch Otolaryngol Head Neck Surg. 1995;121:392-396.
FREE FULL TEXT
11. Molstad S, Arvidsson E, Eliasson I, et al. Production of beta-lactamase in respiratory tract bacteria in children. Scand J Prim Health Care. 1992;10:16-20.
PUBMED
12. Hurley RE, Freund DA, Paul JE. Managed Care in Medicaid: Lessons for Policy and Program Design. Ann Arbor, Mich: Health Administration Press; 1993.
13. St Anthony's Color-Coded ICD-9-CM Book for Physician Payment: Volumes I & II, 1994. Alexandria, Va: St Anthony Publishing Inc; 1993.
14. Halfon N, Newacheck PW. Childhood asthma and poverty: differential impacts and utilization of health services. Pediatrics. 1993;91:56-61.
FREE FULL TEXT
15. Physicians' Current Procedural Terminology: CPT 1994. Chicago, Ill: American Medical Association; 1994.
16. McCaig LF, Hughes JM. Trends in antimicrobial drug prescribing among office-based physicians in the United States. JAMA. 1995;273:214-219.
FREE FULL TEXT
17. Gaffey MJ, Gwaltney JM, Sastre A, et al. Intranasally and orally administered antihistamine treatment of experimental rhinovirus colds. Am Rev Respir Dis. 1987;136:556-560.
WEB OF SCIENCE
| PUBMED
18. Howard JC, Kantner TR, Lillenfield LS, et al. Effectiveness of antihistamines in the symptomatic management of the common cold. JAMA. 1979;242:2414-2417.
FREE FULL TEXT
19. Hueston WJ, Mainous III AG, Farrell JB. Childhood immunization availability in primary care practices. Arch Fam Med. 1994;3:605-609.
FREE FULL TEXT
20. Mainous III AG, Hueston WJ. Medicaid free distribution programs and the availability of childhood immunizations in rural practices. Fam Med. 1995;27:166-169.
PUBMED
21. Williams JW Jr, Holleman DR Jr, Samsa GP, Simel DL. Randomized controlled trial of 3 vs 10 days of trimethoprim/sulfamethoxazole for acute maxillary sinusitis. JAMA. 1995;273:1015-1021.
FREE FULL TEXT
22. Little PS, Williamson I. Are antibiotics appropriate for sore throats? costs outweigh the benfits. BMJ. 1994;309:1010-1011.
FREE FULL TEXT
23. Vinson DC, Lutz LJ. The effect of parental expectations on the treatment of children with a cough: a report from ASPN. J Fam Pract. 1993;37:23-27.
WEB OF SCIENCE
| PUBMED
24. Duchin JS, Breiman RF, Diamond A, et al. High prevalence of multidrug-resistant Streptococcus pneumoniae among children in a rural Kentucky community. Pediatr Infect Dis J. 1995;14:745-750.
WEB OF SCIENCE
| PUBMED
25. Boken DJ, Chartrand SA, Goering RV, Kruger R, Harrison CJ. Colonization with penicillin-resistant Streptococcus pneumoniae in a child-care center. Pediatr Infect Dis J. 1995;14:879-884.
WEB OF SCIENCE
| PUBMED
26. Grimshaw JM, Russell IT. Effect of clinical guidelines on medical practice: a systematic review of rigorous evaluations. Lancet. 1993;342:1317-1322.
FULL TEXT
|
WEB OF SCIENCE
| PUBMED
27. Davis DA, Thomson MA, Oxman AD, Haynes RB. Changing physician performance. JAMA. 1995;274:700-705.
FREE FULL TEXT
28. Schaffner W, Ray WA, Federspiel CF, Miller WO. Improving antibiotic prescribing in office practice. JAMA. 1983;250:1728-1732.
FREE FULL TEXT
29. Greco PJ, Eisenberg JM. Changing physicians' practices. N Engl J Med. 1993;329:1271-1274.
FULL TEXT
|
WEB OF SCIENCE
| PUBMED
30. Avorn J, Soumerai SB. Improving drug-therapy decisions through educational outreach. N Engl J Med. 1983;308:1457-1463.
WEB OF SCIENCE
| PUBMED
31. Shapiro DW, Lasker RD, Bindman AB, Lee PR. Containing costs while improving quality of care: the role of profiling and practice guidelines. Annu Rev Public Health. 1993;14:219-241.
FULL TEXT
|
WEB OF SCIENCE
| PUBMED
32. Schoenbaum SC. Feedback of clinical performance information. HMO Pract. 1993; 7:5-11.
33. Spector SL. The common cold: current therapy and natural history. J Allergy Clin Immunol. 1995;95:1133-1138.
FULL TEXT
|
WEB OF SCIENCE
| PUBMED
RELATED ARTICLE
Physician Heal Thyself: Are Antibiotics the Cure or the Disease?
James P. Richardson
Arch Fam Med. 1998;7(1):51-52.
FULL TEXT
THIS ARTICLE HAS BEEN CITED BY OTHER ARTICLES
 |
Availability of Antibiotics for Purchase Without a Prescription on the Internet
Mainous et al.
Ann Fam Med 2009;7:431-435.
ABSTRACT
| FULL TEXT
Intervention with educational outreach at large scale to reduce antibiotics for respiratory tract infections: a controlled before and after study
Smeets et al.
Fam Pract 2009;26:183-187.
ABSTRACT
| FULL TEXT
Procalcitonin-Guided Antibiotic Use vs a Standard Approach for Acute Respiratory Tract Infections in Primary Care
Briel et al.
Arch Intern Med 2008;168:2000-2007.
ABSTRACT
| FULL TEXT
Antibiotic Prescribing for Children With Nasopharyngitis (Common Colds), Upper Respiratory Infections, and Bronchitis Who Have Health-Professional Parents
Huang et al.
Pediatrics 2005;116:826-832.
ABSTRACT
| FULL TEXT
Effectiveness of a multiple intervention to reduce antibiotic prescribing for respiratory tract symptoms in primary care: randomised controlled trial
Welschen et al.
BMJ 2004;329:431.
ABSTRACT
| FULL TEXT
Antibiotics for acute respiratory tract symptoms: patients' expectations, GPs' management and patient satisfaction
Welschen et al.
Fam Pract 2004;21:234-237.
ABSTRACT
| FULL TEXT
Misconceptions About Colds and Predictors of Health Service Utilization
Lee et al.
Pediatrics 2003;111:231-236.
ABSTRACT
| FULL TEXT
Economic Impact of Influenza Vaccination in Preschool Children
Cohen and Nettleman
Pediatrics 2000;106:973-976.
ABSTRACT
| FULL TEXT
Diagnosis of Attention-Deficit/Hyperactivity Disorder and Use of Psychotropic Medication in Very Young Children
Rappley et al.
Arch Pediatr Adolesc Med 1999;153:1039-1045.
ABSTRACT
| FULL TEXT
Antibiotics for Upper Respiratory Tract Infections: Follow-up Utilization and Antibiotic Use
Hueston et al.
Arch Fam Med 1999;8:426-430.
ABSTRACT
| FULL TEXT
Bacterial Resistance Due to Antimicrobial Drug Addiction Among Physicians: Time for a Cure!
Abramson and Givner
Arch Fam Med 1999;8:79-80.
FULL TEXT
Antibiotics for Colds in Children: Who Are the High Prescribers?
Mainous III et al.
Arch Pediatr Adolesc Med 1998;152:349-352.
ABSTRACT
| FULL TEXT
Physician Heal Thyself: Are Antibiotics the Cure or the Disease?
Richardson
Arch Fam Med 1998;7:51-52.
FULL TEXT
|