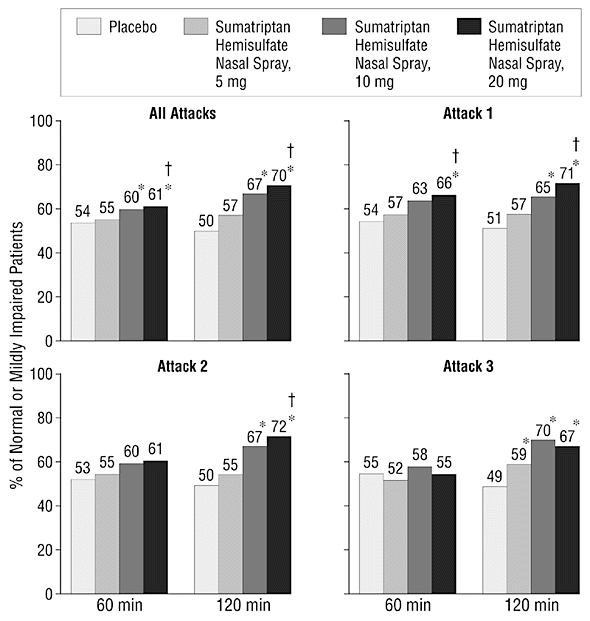
Figure 2. Percentage of patients with no or mild clinical disability 60 or 120 minutes after dosing with sumatriptan hemisulfate nasal spray (5, 10, or 20 mg) or placebo. Asterisk indicates P<.05 vs placebo; dagger, P<.05 vs 5 mg of sumatriptan hemisulfate nasal spray.