 |
 |

A Patient-Initiated System for Preventive Health Care
A Randomized Trial in Community-Based Primary Care Practices
Robert B. Williams, MD;
Myde Boles, PhD;
Robert E. Johnson, PhD
Arch Fam Med. 1998;7:338-345.
ABSTRACT
Objective To test the effectiveness of a patient-initiated, touch-sensitive computer system (TSCS) for improving screening rates for cancers of the breast, cervix, colon and rectum, and oral cavity.
Design One-year, randomized, controlled trial with primary care practice as the unit of analysis.
Setting Sixty primary care practices, randomly recruited from 329 nonteaching practices in a southeastern state.
Subjects Random sample of the medical records of 50 male and female adult patients before intervention and 50 adult patients after intervention in each practice and a random sample of 507 TSCS users.
Interventions Touch-sensitive computer system and a registered nurse who served as liaison to the study practices. The TSCS provided patient-specific preventive service recommendations and facilitated work flow to increase the completion of these interventions.
Main Outcome Measure Average change, adjusted for health maintenance examination (HME) and use of the TSCS, in the proportion of eligible patients undergoing screening mammography, clinical breast examination, digital rectal examination, fecal occult blood test, flexible sigmoidoscopy, Papanicolaou smear, and oral cavity examination.
Results We observed a significant increase in the completion of screening mammography (6.6%; P .05) and clinical breast examination (6.1%; P .05) and clinical breast examination (6.1%; P .01) in women 50 years of age and older, particularly for those who had an HME during the study year. .01) in women 50 years of age and older, particularly for those who had an HME during the study year.
Conclusions Patients who have HMEs are more likely to receive cancer screening; however, a computer-based system for preventive services can contribute to improvement in screening. Among those patients who did not have an HME, TSCS users had higher rates of breast cancer screening than nonusers.
INTRODUCTION
IT IS WIDELY recognized that the delivery of clinical preventive services in primary care practice falls well below recommended levels. Barriers to implementing preventive services persist, despite an abundance of studies on ways to overcome them.1-3 The most frequently cited barriers are lack of time, daily demands of managing a practice, disease orientation of medical education and practice, insufficient provider knowledge, lack of reimbursement, and forgetfulness.2, 4 A recent report by the American Cancer Society Advisory Group on Preventive Health Care Reminder Systems states that the most important reason for failure to provide preventive services adequately is "the lack of a systematic, organized approach within the office."3
Historically, the wide variety of preventive care interventions were studied mostly in academic settings and were focused primarily on single strategies to improve delivery of preventive services, ie, physician education, physician feedback and audit, medical chart reminders, and flow sheets for preventive services.1-2,5 The results of these earlier studies are mixed, although many show some incremental increase in the delivery of selected preventive services in the short run. More recently, integrated, systematic approaches have been tried and have shown improvement over earlier, single-intervention strategies.6-7
Clinicians provide more preventive services to patients who visit for a health maintenance examination (HME).8-11 However, most adult patients do not seek HMEs, and the opportunity to deliver preventive care is curtailed. Consequently, consensus groups, experts, and researchers recommend restructuring the primary care environment to incorporate preventive services into routine care.1, 12-15
Interventions to improve the delivery of preventive services must address the complexity and magnitude of prevention recommendations while coordinating prevention activities of nurses, office staff, and clinicians with the provision of symptom-driven patient care. We developed a patient-initiated, touch-sensitive computer system (TSCS) for preventive health care services with this goal in mind. We undertook this study to determine whether the TSCS can improve secondary prevention outcomes for cancers of the breast, colon and rectum, cervix, and oral cavity by incorporating a comprehensive preventive care process into the routine care activities of office staff, nursing staff, physicians, and physician extenders (nurse practitioners and physician assistants).
SUBJECTS AND METHODS
SAMPLE
A stratified, 2-stage cluster sampling design was used to gather data on primary care practices (family practice, general practice, and internal medicine) and their patient populations. At the first stage, 60 primary care practices (primary sampling units) were randomly recruited from a population of 329 nonteaching primary care practices in a 43-county area in Virginia. Before recruitment, practices were stratified according to their degree of linkage with the Medical College of Virginia, Virginia Commonwealth University, Richmond (MCV-VCU) (high-link practices were those with MCV-VCU family practice residency graduates, with physicians who served as preceptors for medical students, or with local opinion leaders who served on MCV-VCU committees; low-link practices were those with no such connections). We also stratified practices according to whether they subscribed to the Virginia Insurance Reciprocal (VIR) for malpractice insurance. The VIR agreed to provide a 1-time 6% annual premium reduction to physicians in practices that incorporated the TSCS into patient care. The stratification scheme resulted in the following 4 strata consisting of 12 practices each: high-link VIR; high-link without VIR; low-link VIR; and low-link without VIR. A fifth stratum consisted of 12 federally funded community health centers (CHCs) under the Virginia Primary Care Association, Inc. Practices were recruited in random order, within a stratum and between strata, until 12 practices were identified in each stratum that agreed to participate in the study. Practices were then randomized to intervention or control within each stratum. One CHC intervention practice withdrew from the study due to loss of personnel; another CHC control practice withdrew due to the loss of its designation as a CHC. Twenty-nine intervention practices received the TSCS plus the support of a nurse liaison for 12 months. Twenty-nine control practices had the opportunity to receive the TSCS after the 12-month study with 3 months of nurse liaison support.
At the second stage, 50 patient medical records (secondary sampling units) were randomly sampled from each practice's adult patient population before implementation of the intervention, and 50 records were independently sampled after completion of the intervention. These sample sizes were chosen to achieve 80% power to detect a clinically significant difference of 10% in screening rates. Schematics of each practice's chart room were drawn and blocked into sections of charts. Twenty sections at a time were randomly selected, and a random offset, a linear measure in centimeters, was chosen. The purpose of the offset was to avoid the increased probability of selecting fat medical records. In each section, the first chart beyond the offset was selected. This procedure was repeated until the 50 eligible charts had been selected.
The Committee on the Conduct of Human Research at Virginia Commonwealth University approved the study.
INTERVENTION
The intervention consisted of the TSCS and a registered nurse detailer who served as liaison to the study practices. The TSCS was designed to provide patient-specific preventive care recommendations and to facilitate work flow in the primary care practice environment in an effort to increase the completion of high-priority adult preventive care interventions. The TSCS was not designed to assess health risk for mortality or morbidity.
The TSCS intervention begins with the patient. While in the waiting room, the patient touches the computer screen and answers 20 to 25 questions on personal medical history, family medical history, and lifestyle. The computer prints out patient-specific physician chart reminders, chart organizers, order sheets, and patient education materials at the reception area, where the receptionist then places them on the front of the patient's medical record. The 1-page chart reminder provides the physician with an overview of all the patient's preventive needs. Optimally, all clinicians and office staff have a role in the TSCS process. The TSCS is also capable of producing reports on individual patients' preventive care status, reminder letters, and summary reports on the practice's overall preventive care activities. The system simply requires turning the power on daily and keeping a full supply of paper in the printer. The system resets itself after each user.
Prevention recommendations on cancer, coronary artery disease, smoking, osteoporosis, immunizations, occupational noise exposure, seatbelt and shoulder harness use, and substance abuse were derived from several sources. We drew on the health care literature; published guidelines of the American Cancer Society, the National Cancer Institute, and the United States Preventive Services Task Force; and experienced practitioner expertise to identify a core of appropriate adult health promotion and disease preventive care interventions. They were designed to be pragmatic and were based on what primary care physicians would do if they had the desired time to provide those services to all patients. Before conducting this randomized trial, we pilot-tested the TSCS using patient focus groups and conducted a randomized validation study to assess the accuracy of the patient data collection. Patients' responses to the TSCS questions were highly sensitive and specific, compared with clinician-obtained histories. Overall error rate (sensitivity and specificity errors divided by the total number of responses) for cancer-related questions was only 6.3%. These results were reported previously.16
Three registered nurses served as liaisons to the 58 study practices. Each nurse was randomly assigned responsibility for an equal number of intervention and control practices. The nurses worked as detailers for the TSCS, initiating the change process and demonstrating the benefits of the system. They developed and distributed resource manuals that contained TSCS information and community and MCV-VCU information on cancer prevention services and patient education. The nurses were readily available to meet practice needs during implementation.
All intervention practices received an initial intensive, half-day training session conducted by the nurse detailer assigned to the practice and one of us (R.B.W.). All clinicians and office staff were strongly encouraged to attend, and attendance level was high. The training session emphasized using the TSCS in a manner compatible with current practice environment and culture. We did not mandate 1 way to use the system, but we strongly urged practices to place the computer where it would be accessible to all patients, preferably in the waiting room. We also encouraged clinicians to delegate TSCS-generated preventive care activities to nurses and other office staff, as appropriate.
DATA COLLECTION
Patients' medical records were eligible for review if the patient was 18 years of age or older and had visited the practice in the previous year. From the medical records, we obtained baseline data on patients eligible for the following selected cancer screening activities performed or completed in the year before the intervention: screening mammography for women 50 years of age and older, clinical breast examinations for women 50 years of age and older, Papanicolaou smears for women 18 years of age and older, digital rectal examinations for men and women 40 years of age and older, fecal occult blood tests for men and women 50 years of age and older, flexible sigmoidoscopies for men and women 50 years of age and older, and oral cavity examinations for men and women 18 years of age and older. We were interested not only in the presence or absence of these activities, but also in their dates of occurrence, whether the patient was indicated for a particular screening test according to guidelines, and whether the patient had had an HME during the year. An HME was defined as an office visit expressly for the purpose of a physical examination, breast examination, Papanicolaou smear and pelvic examination, or annual check-up. We repeated our data collection after the 1-year intervention.
We collected data entered by 9858 adult patients who used the TSCS during the study year. In each of the intervention practices, we obtained a random sample of up to 20 TSCS users for the purpose of reviewing their medical records to determine if the TSCS influenced their completion of cancer screening activities compared with nonusers. We also conducted telephone interviews on an independent random sample of 60 TSCS users in each practice to obtain information on patient satisfaction and ease of use. The results of the patient interviews are reported elsewhere.16
Before the intervention, we mailed a 65-item survey to the clinical or office administrator at each practice to obtain data on practice characteristics (eg, number of full-time equivalent [FTE] clinicians) and prevention policies (eg, written policy for prevention). Fifty-four (93%) of the 58 practices returned the survey.
STATISTICAL ANALYSIS
The 329 primary care practices in the sampling frame and their patient populations were defined as the universe; the practice was the unit of analysis. The proportions of patients eligible for completing cancer screening activities in the preintervention and the intervention years were the parameters about which we made inference. We obtained estimates and associated SEs of these proportions using a statistical method that adjusted for the disproportionate sampling sizes across strata (sampling weights) and for the sampling of practices without replacement from a small population (sampling fractions).17
The main effect of the intervention was measured by the average change in the proportion of eligible patients completing each cancer screening test in the intervention practices, compared with the average change in the control practices. These comparisons were also adjusted by the occurrence of an HME during the study year and patients' use of TSCS.
Logistic regression was performed to determine the effect of the intervention on the probability of screening in the presence of various practice and patient characteristics. We specified a logistic regression model for each cancer screening test. Sampling weights (relative sizes of the strata) were used to represent the relative value of each practice's contribution to the aggregate model estimates. The SEs of the model estimates were adjusted for sampling from a small, finite population of practices. Because patient populations were large relative to within-practice patient sample size, we did not correct for sampling of finite patient populations at this stage. The logistic module of the software package SUDAAN 6.0 (Research Triangle Institute, Research Triangle Park, NC) was used to account for the complex sampling design involving practice variation and patient variation within practices.
The dependent variable in each logistic regression was an indicator (0,1) of completion of the cancer screening test in question. Each model included the following set of core explanatory variables representing the basic structure of the study design: study group (intervention or control practice), time (preintervention or postintervention), and the interaction between study group and time. The interaction term was included in the model to indicate the effect of the intervention (ie, where the difference in the proportion of screening between intervention and control practices in the postintervention period was greater than the difference in the preintervention period).
We examined interactions between the core explanatory variables and covariables in separate logistic regression models. These interactions allowed for a different core model to be fit for each level of a covariable. Interactions were deemed significant, using a backward elimination procedure, if the associated P value was less than or equal to .10 ( =.10). Although some significant interactions were found, none contributed to our better understanding the effect of the intervention. =.10). Although some significant interactions were found, none contributed to our better understanding the effect of the intervention.
RESULTS
During the study year, 9858 regular adult patients in 29 intervention practices used the TSCS. One intervention practice and 1 control practice withdrew from the study during the year. Both practices were in the CHC stratum. We reviewed the medical records of 5789 eligible patients, 18 years of age and older, plus an additional sample of 507 TSCS users. Characteristics of the study practices are presented in the following tabulation:
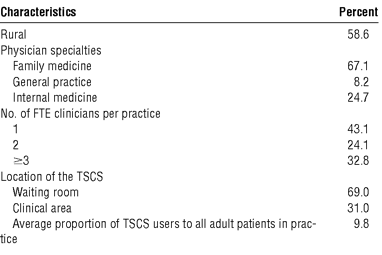
On average, the study practices had 2.3 FTE clinicians. Twenty of the 29 intervention practices opted to put the TSCS in the waiting room. The remaining practices put the system in a clinical area, where it was used by a selected group of patients as determined by the practice.
Overall, we observed a significant increase in the completion of screening mammography and clinical breast examinations in women 50 years of age and older in the intervention group practices. Proportions of patients undergoing screening for each cancer are shown in Table 1 and are compared for preintervention and postintervention periods. For all screening tests except fecal occult blood test, the change in completed screening tests in the intervention group was positive and larger than the change in control practices; however, the differences were significant (P .05) only for screening mammography and clinical breast examination. Notably, the proportion of women in intervention practices who received mammography increased by 26.8%, and the proportion of women who received clinical breast examinations increased by 20.3%. .05) only for screening mammography and clinical breast examination. Notably, the proportion of women in intervention practices who received mammography increased by 26.8%, and the proportion of women who received clinical breast examinations increased by 20.3%.
|
|

|
|
Table 1. Comparison of Change in Screening Rates Between Intervention and Control Practices
|
|
|
We were interested in the contribution of the HME to the completion of cancer screening tests. In the preintervention period, 21.7% of patients had HMEs (control practices, 21.1%; intervention practices, 22.1%). In the postintervention period, 25.8% of patients had HMEs (control practices, 26.5%; intervention practices, 26.1%). Table 2 compares changes in screening rates between intervention and control practices for patients who did and did not have an HME during the study year. For patients who had an HME during the study year, the change in screening rates from preintervention to postintervention was significantly larger for mammography, clinical breast examination, and oral cavity examination in the intervention practices, compared with control practices. For patients who did not have an HME during the study year, the difference between intervention and control practices in the change from preintervention to postintervention was not significant. Overall, women who had HMEs in intervention practices showed a significant improvement in completion rates for screening mammography and clinical breast examination.
|
|

|
|
Table 2. Comparison of Change in Screening Rates Between Intervention and Control Practices in Presence of HME*
|
|
|
Finally, we wanted to know whether patients who used the TSCS had higher rates of completion of cancer screening tests than nonusers. Since we ascertained the importance of having an HME, we included presence or absence of HME in the analysis. As shown in Table 3, TSCS use was associated with higher rates of completion overall for all cancer screening tests. Among patients who did not have an HME during the study year, TSCS use was associated with approximately twice as large a proportion of women receiving screening mammography and clinical breast examination, compared with nonusers. We did not, however, find significant differences for the other cancer screening tests between users and nonusers among women who had not had HMEs. Conversely, TSCS users who had an HME during the study year were associated with higher proportions of fecal occult blood tests, flexible sigmoidoscopies, and oral cavity examinations, compared with nonusers who had an HME. The results indicate that, whereas use of the TSCS was associated with higher rates of screening overall, the effect of the intervention was predominant in breast cancer screening for women who did not have HMEs.
|
|

|
|
Table 3. Comparison of Touch-Sensitive Computer System (TSCS) Users With Nonusers, With and Without HME*
|
|
|
The results of the logistic regression procedure for each of the 7 cancer screening tests are presented in Table 4. For each model and variable, we show the odds ratio (OR) that compares the screening probability for the first level of the variable with the screening probability for the second level, except for written policy for preventive care, which has 3 levels. An OR greater than 1.0 means that the screening probability is higher for the first level of that variable. For example, the odds of completing screening mammography are nearly 6 times greater (OR=5.61) for women who had an HME during the study year compared with women who did not, all else held constant.
|
|

|
|
Table 4. Logistic Regression Models for Cancer Screening Tests*
|
|
|
DESIGN VARIABLES
The ORs for the design variables indicate differences in screening probabilities between study and control practices, preintervention and postintervention periods, and the interaction between both variables. The interaction indicates the change from preintervention to postintervention in practices' screening probabilities compared with control practices, which is not explained by other variables in the model. The interaction ORs are marginally significant for both breast cancer screening tests (P .10). When these models are fitted using only the design variables, the ORs correspond to the same screening probabilities shown in Table 1 and have similar magnitudes to the ORs reported in Table 4. .10). When these models are fitted using only the design variables, the ORs correspond to the same screening probabilities shown in Table 1 and have similar magnitudes to the ORs reported in Table 4.
PRACTICE-LEVEL VARIABLES
Rural practices were marginally less likely to complete mammograms and Papanicolaou smears (P .10) than urban and suburban practices, but the completion of fecal occult blood tests was 2.9 times higher in rural practices (P .10) than urban and suburban practices, but the completion of fecal occult blood tests was 2.9 times higher in rural practices (P .05). Practices with 3 or more FTE physicians were significantly more likely to complete mammograms and clinical breast examinations (P .05). Practices with 3 or more FTE physicians were significantly more likely to complete mammograms and clinical breast examinations (P .01). Smaller practices with 1 or 2 FTE physicians were marginally more likely to complete oral cavity examinations (P .01). Smaller practices with 1 or 2 FTE physicians were marginally more likely to complete oral cavity examinations (P .10). .10).
Among those practices that responded to the question about a written policy for preventive care, those with a written policy were 3 times more likely to complete oral cavity examinations (P .05). The written policy (yes vs no) ORs for breast and colorectal cancer screening tests were consistently less than 1, but only those for clinical breast examination and flexible sigmoidoscopy reached marginal significance (P .05). The written policy (yes vs no) ORs for breast and colorectal cancer screening tests were consistently less than 1, but only those for clinical breast examination and flexible sigmoidoscopy reached marginal significance (P .10). .10).
The location of the TSCS played a role in the completion of cancer screening tests. For all tests, the location of the system in the patient waiting room was associated with a higher probability of completing screening tests than location in a clinical area. This result was strongly significant for breast cancer screening tests and Papanicolaou smears (P .01) and was significant for digital rectal examinations (P .01) and was significant for digital rectal examinations (P .05). .05).
PATIENT-LEVEL VARIABLES
Colorectal screening and oral cavity screening were performed on both sexes; thus the gender variable was included in the corresponding models. Men were more likely to complete digital rectal examinations and flexible sigmoidoscopy as indicated by an OR significantly greater than 1.0 (P .01). No significant sex differences were observed for fecal occult blood tests and oral cavity examinations. .01). No significant sex differences were observed for fecal occult blood tests and oral cavity examinations.
The strong effect of the HME (P .01) is seen in the logistic regression models across all 7 screening tests, even after accounting for the effects of other model variables. An HME visit during the study year is associated with an increase in screening probability ORs ranging from 1.78 for flexible sigmoidoscopy to 18.20 for clinical breast examination. .01) is seen in the logistic regression models across all 7 screening tests, even after accounting for the effects of other model variables. An HME visit during the study year is associated with an increase in screening probability ORs ranging from 1.78 for flexible sigmoidoscopy to 18.20 for clinical breast examination.
The effect of the TSCS intervention was seen in the design variable interaction between study group and period and the patient-level variable use of the TSCS. Use of the TSCS was associated with an increase in the probability of completing all screening tests (OR>1.00). The ORs for breast and colorectal cancer screening were significantly greater than 1, with marginal significance for digital rectal examination.
COMMENT
We designed our study primarily to detect a change in the overall proportion of patients completing cancer screening tests as a result of the TSCS being incorporated into the practice routine. We found that community-based, primary care physicians achieved significant improvement in the proportion of patients who received screening mammography and clinical breast examination as a result of using the TSCS in their practices. For the other cancer screening tests we studied, we observed improvement (although not statistically significant) in the overall proportion of patients obtaining screening tests in the intervention practices.
Looking closely at the patient factors attributed to screening, we discovered that patients who had an HME during the study year had a substantially higher probability of completing all screening tests, even after taking into account the effect of the intervention. The odds of screening in the presence of HME ranged from nearly 2 times greater for flexible sigmoidoscopy to 18 times greater for clinical breast examination. We were not surprised by this finding, since it is well established in the literature that busy practitioners find little time other than at designated HMEs to incorporate preventive services into routine patient care.8-11 However, despite the strong influence of the HME, we were still able to detect improvement in cancer screening rates for patients who used the TSCS. Notably, women who did not have an HME but did use the TSCS experienced higher completion levels of screening mammography and clinical breast examination than those who did not use the TSCS. This suggests that certain patients who do not initiate health maintenance can still be incorporated into the cancer screening process with a computer system like the TSCS. The TSCS was designed specifically to give providers an opportunity to initiate preventive care with patients who are not health maintenance seekers. This has been described as opportunistic screening, and has been demonstrated to be an effective solution to the dilemma of implementing preventive services in primary care.2
We designed our study to determine the effect of the intervention on overall practice-level screening rates. We found that the presence of the TSCS in intervention practices was associated with a significantly higher proportion of women receiving mammography and clinical breast examinations. However, we also discovered that the odds of receiving all of the designated cancer screening tests were significantly improved by the presence of the HME, and that a larger proportion of TSCS users completed all screening tests relative to nonusers. We suspect a degree of interdependence between HME and TSCS use, ie, patients who visited the practice for HMEs were more likely to be asked by clinicians and staff to use the TSCS, or that TSCS users were a self-selected group of patients more inclined to engage in preventive activities. It is only with breast cancer screening that we detected a significant TSCS effect independent of HME status.
Our intervention appears to have had the most impact on the overall practice routine for those well-established screening tests about which women and clinicians have considerable awareness. We were disappointed that our intervention did not lead to practice-level improvement in other screening tests for which there was much more room to improve. Because the TSCS was designed to address a comprehensive set of preventive care recommendations, the system may have overwhelmed some clinicians. As a result, they may have selected only the most well-established recommendations that could be applied to the most frequently visiting patient population.
In busy primary care practices where physicians must attend to many competing demands,18 finding opportunities to incorporate prevention into routine care is challenging. Our system was designed to be more than a specific tool (eg, a flow sheet or chart reminder system). It involved the entire primary care practice and addressed many of the key activities for developing and implementing office systems for preventive services proposed by the American Cancer Society Advisory Group on Preventive Health Care Reminder Systems.3 We recognized the complexity of the implementation process and attempted to tailor the system to each practice's existing organizational structure, formal and informal decision-making processes, patterns of communication, and task coordination. The nature of the relationship between the academic research center and the community primary care practice required sensitivity and flexibility in the implementation process. We addressed these issues by allowing each practice to decide who would coordinate the system, where the system would be placed, and what elements of the system they would use. Perhaps, had we focused more on the strategy for implementation (eg, continuous quality improvement, academic detailing, reengineering), we might have had greater success in improving the delivery of all targeted cancer prevention services.
A limitation of our study was the inability to associate the HME with the visit in which the screening was performed. Our measure, the presence of an HME during the study year, does not provide a causal connection between an HME and the screening tests performed during that visit; however, our study design did allow us to detect this strong association during the study year. A second limitation of our study was budgetary. We used the liaison research nurses as chart reviewers. As a result, our outcome measures could be biased, because the nurses were not blind to the intervention, and because they had a stake in the study outcome. We attempted to account for potential bias in 2 ways. First, we conducted an interrater reliability study on preintervention chart reviews. We did not detect any substantial differences among our 3 study nurses. If any bias existed, it was shared by all 3 in the same magnitude and direction. Second, we hired an independent reviewer to conduct a review of a sample of charts. We did not find any significant differences. A third limitation was the short duration of the 1-year intervention. A longer period would have allowed the TSCS to become institutionalized into the practice and create greater opportunities to improve cancer screening. Finally, the measures of completed cancer screening tests in the postintervention period obtained from patients' medical records may have been inflated because of test reaction bias.19 This occurs in any study where merely conducting the chart reviews in the medical office sensitizes the clinicians and staff to document screening activities more consistently. In our study, the test reaction bias would be found in intervention and control practices. This means that the magnitude of the change in documented screening rates from preintervention to postintervention may be larger than actually occurred, but that the relative difference in this change between interventions and controls would remain the same.
As managed care moves into traditionally nonmanaged care settings, the demand and requirement for delivering more preventive services in primary care will continue to be frustrated by a myriad of barriers, unless a systematic approach is taken to help the busy clinician achieve his or her preventive care goals. Managed care plans that want to compete successfully for health care purchasers must be sensitive to the complexity of delivery of preventive services and support well-designed implementation strategies for comprehensive office systems for preventive care. Likewise, clinicians who desire to increase the delivery of preventive care to their patients must use strategies that successfully incorporate this care into the routine of their current practice. Systematic interventions are most likely to improve preventive care activities in those patients who are already inclined to seek health maintenance care; however, with focused efforts, physicians and their staffs should be able to provide preventive services to nonseekers as well.
AUTHOR INFORMATION
Accepted for publication October 27, 1997.
This study was supported by grant R01-CA54345 from the National Cancer Institute, Bethesda, Md.
We thank Jeffry Reihl, MD, for research assistance; and Corliss Booker, RN, Amy Burgett, RN, and Shawn Gannon, RN, for primary data collection.
Reprints: Robert B. Williams, MD, Ernst & Young, LLP, 901 E Cary St, Suite 1000, Richmond, VA 23219 (e-mail: robert.williams12{at}ey.com).
From the Department of Family Practice, Medical College of Virginia, Virginia Commonwealth University, Richmond. Dr Williams is now affiliated with Ernst & Young, LLP, Richmond; Dr Boles, with the Department of Family Practice, the Center for Health Research, Kaiser Permanente Northwest Division, Portland, Ore.
REFERENCES
1. Hahn DL, Berger MG. Implementation of a systematic health maintenance protocol in a private practice. J Fam Pract. 1990;31:492-504.
WEB OF SCIENCE
| PUBMED
2. Pommerenke FA. Implementing preventive services: practical strategies for primary care physicians. Cancer Prev. 1992;3:1-13.
3. Leininger LS, Finn L, Dickey L, et al. An office system for organizing preventive services: a report by the American Cancer Society Advisory Group on Preventive Health Care Reminder Systems. Arch Fam Med. 1996;5:108-115.
FREE FULL TEXT
4. McGinnis MJ, Griffith HM. Put prevention into practice: a systematic approach to the delivery of clinical preventive services. Arch Intern Med. 1996;156:130-132.
FREE FULL TEXT
5. McPhee SJ, Bird JA, Fordham D, Rodnick JE, Osborn EH. Promoting cancer prevention activities by primary care physicians. JAMA. 1991;266:538-544.
FREE FULL TEXT
6. Gemson DH, Ashford AR, Dickey LL, et al. Putting prevention into practice: impact of a multifaceted physician education program on preventive services in the inner city. Arch Intern Med. 1995;155:2210-2216.
FREE FULL TEXT
7. Dietrich AJ, O'Connor GT, Keller A, Carney PA, Levy D, Whaley FS. Cancer: improving early detection and prevention: a community practice randomised trial. BMJ. 1992;304:687-691.
8. Orleans CT, George LK, Houpt JL, Brodie KH. Health promotion in primary care: a survey of US family practitioners. Prev Med. 1985;14:636-647.
FULL TEXT
|
WEB OF SCIENCE
| PUBMED
9. Mandel IG, Franks P, Dickinson JC. Screening guidelines in a family medicine program: a five-year experience. J Fam Pract. 1982;14:901-907.
PUBMED
10. Dietrich AJ, Goldberg H. Preventive content of adult primary care: do generalists and subspecialists differ? Am J Public Health. 1984;74:223-227.
WEB OF SCIENCE
| PUBMED
11. McPhee SJ, Richard RJ, Solkowitz SN. Performance of cancer screening in a university general internal medicine practice: comparison with the 1980 American Cancer Society guidelines. J Gen Intern Med. 1986;1:275-281.
WEB OF SCIENCE
| PUBMED
12. Battista RN, Lawrence RS. Implementing preventive services. Am J Prev Med. 1988;4:53-67.
PUBMED
13. Battista RN. Adult cancer prevention in primary care: patterns of practice in Quebec. Am J Public Health. 1983;73:1036-1039.
WEB OF SCIENCE
| PUBMED
14. Frame PS. Health maintenance in clinical practice: strategies and barriers. Am Fam Physician. 1992;45:1192-1200.
WEB OF SCIENCE
| PUBMED
15. United States Preventive Services Task Force. Guide to Clinical Preventive Services: An Assessment of the Effectiveness of 169 Interventions. Baltimore, Md: Williams & Wilkins; 1989.
16. Williams RB, Boles M, Johnson RE. Patient use of a computer for prevention in primary care practice. Patient Educ Couns. 1995;25:283-292.
FULL TEXT
|
WEB OF SCIENCE
| PUBMED
17. Cochran WG. Sampling Techniques. New York, NY: John Wiley & Sons Inc; 1977:274-280.
18. Jaen CR, Strange KC, Nutting PA. Competing demands of primary care: a model for the delivery of clinical preventive services. J Fam Pract. 1994;38:166-171.
WEB OF SCIENCE
| PUBMED
19. Campbell DT, Stanley JC. Experimental and Quasi-experimental Designs for Research. Chicago,ll: Rand-McNally; 1963:9.
RELATED ARTICLES
Cancer Screening: A Challenge in Today's Changing Practice of Medicine
Roselyn Payne Epps
Arch Fam Med. 1998;7(4):315-316.
FULL TEXT
Can We Change Physicians' Practices in the Delivery of Cancer-Preventive Services?
Mack T. Ruffin IV
Arch Fam Med. 1998;7(4):317-319.
FULL TEXT
Cancer Early-Detection Services in Community Health Centers for the Underserved: A Randomized Controlled Trial
Allen J. Dietrich, Jonathan N. Tobin, Carol Hill Sox, Andrea N. Cassels, Fredeswinda Negron, Richard G. Younge, Neal A. Demby, and Tor D. Tosteson
Arch Fam Med. 1998;7(4):320-327.
ABSTRACT
| FULL TEXT
Prescribe for Health: Improving Cancer Screening in Physician Practices Serving Low-Income and Minority Populations
Clara Manfredi, Ronald Czaja, Sally Freels, Mitchell Trubitt, Richard Warnecke, and Loretta Lacey
Arch Fam Med. 1998;7(4):329-337.
ABSTRACT
| FULL TEXT
THIS ARTICLE HAS BEEN CITED BY OTHER ARTICLES
 |
Prompting Clinicians about Preventive Care Measures: A Systematic Review of Randomized Controlled Trials
Dexheimer et al.
J Am Med Inform Assoc 2008;15:311-320.
ABSTRACT
| FULL TEXT
Delivery of Cancer Screening: How Important Is the Preventive Health Examination?
Fenton et al.
Arch Intern Med 2007;167:580-585.
ABSTRACT
| FULL TEXT
Systematic Review: The Value of the Periodic Health Evaluation
Boulware et al.
ANN INTERN MED 2007;146:289-300.
ABSTRACT
| FULL TEXT
Assessment of an Interactive Computer-Based Patient Prenatal Genetic Screening and Testing Education Tool
Griffith et al.
Health Educ Behav 2005;32:613-626.
ABSTRACT
Informed patient consent to participation in cluster randomized trials: an empirical exploration of trials in primary care
Eldridge et al.
Clin Trials 2005;2:91-98.
ABSTRACT
A Randomized Controlled Trial to Increase Cancer Screening Among Attendees of Community Health Centers
Roetzheim et al.
Ann Fam Med 2004;2:294-300.
ABSTRACT
| FULL TEXT
Cancer Prevention Services and Physician Consensus in Primary Care Group Practices
Love et al.
Cancer Epidemiol. Biomarkers Prev. 2004;13:958-966.
ABSTRACT
| FULL TEXT
Effectiveness of Interventions to Increase Papanicolaou Smear Use
Yabroff et al.
J Am Board Fam Med 2003;16:188-203.
ABSTRACT
| FULL TEXT
Using patient-driven computers to provide cost-effective prevention in primary care: a conceptual framework
Shakeshaft and Frankish
HEALTH PROMOT INT 2003;18:67-77.
ABSTRACT
| FULL TEXT
The Patient-Computer Interview: A Neglected Tool That Can Aid the Clinician
Bachman
Mayo Clin Proc. 2003;78:67-78.
ABSTRACT
Computer-generated Patient Education Materials: Do They Affect Professional Practice?: A Systematic Review
Treweek et al.
J Am Med Inform Assoc 2002;9:346-358.
ABSTRACT
| FULL TEXT
Explaining Physician Rates of Providing Flexible Sigmoidoscopy
Montano et al.
Cancer Epidemiol. Biomarkers Prev. 2000;9:665-669.
ABSTRACT
| FULL TEXT
Effectiveness of Interventions Designed to Increase Mammography Use: A Meta-Analysis of Provider-targeted Strategies
Mandelblatt and Yabroff
Cancer Epidemiol. Biomarkers Prev. 1999;8:759-767.
ABSTRACT
| FULL TEXT
A patient initiated computer program improved breast cancer screening practices in primary care
Kernohan
Evid. Based Nurs. 1999;2:57-57.
FULL TEXT
Can we change physicians' practices in the delivery of cancer-preventive services?
Epps
Arch Fam Med 1998;7:315-316.
FULL TEXT
Can We Change Physicians' Practices in the Delivery of Cancer-Preventive Services?
Ruffin IV
Arch Fam Med 1998;7:317-319.
FULL TEXT
|