 |
 |

Prognostic Factors for Persons With Idiopathic Chronic Fatigue
Arthur J. Hartz, MD, PhD;
Evelyn M. Kuhn, PhD;
Suzanne E. Bentler, MS;
Paul H. Levine, MD;
Richard London, MD
Arch Fam Med. 1999;8:495-501.
ABSTRACT
 |  |
Background The simultaneous examination of a large number of patient characteristics in a prospective study of patients with chronic fatigue.
Objective To compare the relative importance of these characteristics as prognostic factors.
Methods The data analyzed were from 199 subjects in a registry of persons who were aged 18 years or older and had idiopathic fatigue for at least 6 months. All subjects completed an extensive baseline questionnaire that provided information about fatigue, demographic characteristics, medical conditions, lifestyle, sleeping habits, psychological characteristics, and the presence of criteria for chronic fatigue syndrome. Changes in fatigue severity from baseline to 2-year follow-up were tested for an association with risk factors at baseline and with changes in symptoms other than fatigue during the follow-up period.
Results The following characteristics at baseline significantly and independently predicted greater fatigue improvement: less unclear thinking, fewer somatoform symptoms not used to define chronic fatigue syndrome, infrequent awakening, fewer hours sleeping, and being married. Of 29 subjects who at baseline reported no somatoform symptoms unrelated to chronic fatigue syndrome and who thought clearly most of the time, 8 substantially improved, compared with 1 of 29 subjects who had more than 2 somatoform symptoms and never thought clearly (P = .01). Improvements in the following symptoms were significantly and independently associated with improvements in fatigue: unclear thinking, depression, muscle aches, and trouble falling asleep.
Conclusions This study identified characteristics of subjects that seem to be of prognostic importance for idiopathic chronic fatigue. Symptoms that change concomitantly with changes in fatigue may be intrinsically linked to fatigue.
INTRODUCTION
FATIGUE HAS a "powerful adverse effect on quality of life,"1 and is a primary reason for seeking medical care. Treatment of fatigue, however, is often ineffective2-4 and the problem tends to persist. Studies have found that 58% to 72% of persons continue to have fatigue after 1 year,5-8 and 1 study found that 59% still had fatigue after 2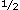 years.8 To devise better treatments, it would be helpful to understand both the factors that initiate fatigue and the factors that prolong fatigue. These latter factors may contribute to prolonging fatigue regardless of the cause just as anxiety, lower activity levels, and catastrophizing contribute to the prolongation of chronic pain regardless of the cause.9-10 Identifying these factors is a first step in developing an effective treatment for patients with fatigue. years.8 To devise better treatments, it would be helpful to understand both the factors that initiate fatigue and the factors that prolong fatigue. These latter factors may contribute to prolonging fatigue regardless of the cause just as anxiety, lower activity levels, and catastrophizing contribute to the prolongation of chronic pain regardless of the cause.9-10 Identifying these factors is a first step in developing an effective treatment for patients with fatigue.
Previous studies have examined the association between patient prognosis and several risk factors. This study complements the previous work in 4 ways: (1) It evaluated a large number of risk factors in the same study population so the relative importance of the risk factors could be compared. (2) The types of subjects studied differed from previous study samples. (3) Some risk factors examined had been studied infrequently or not at all. (4) Evidence of an intrinsic association between the risk factor and fatigue was examined by testing the association between changes in fatigue and changes in the risk factor.
PATIENTS AND METHODS
SUBJECT SELECTION
Data were obtained from a registry of persons older than 17 years who had experienced fatigue for at least 6 months. The collection of the data for the registry was approved by the Human Research Committee of the Medical College of Wisconsin, Milwaukee, during the follow-up period, and all patients who participated in the study signed a consent form. All patients in the registry completed an extensive questionnaire that provided information about fatigue, demographics, medical conditions, lifestyle, sleeping habits, and psychological characteristics. Patients also completed a follow-up questionnaire that provided information on fatigue, sleeping habits, depression, and somatoform symptoms. Subjects were (1) members of the Wisconsin Chronic Fatigue Association, Madison, (2) persons from the community who attended presentations on chronic fatigue syndrome (CFS), or (3) patients in participating medical clinics. Patients from the medical clinics had responded to signs in the clinics that asked for volunteers. These patients were not necessarily being treated for fatigue.
Subjects were excluded from this study if they did not meet our criteria for substantial fatigue as described later in this article. We also excluded subjects who reported other illnesses that might account for their fatigue. The illnesses were determined based on the subjects' responses to a questionnaire that asked whether they had ever been told by a physician that they had any of several specific chronic illnesses or a chronic illness that was not on the list. Subjects who reported the following illnesses were excluded: cancer, diabetes, heart diseases, chronic bronchitis or emphysema, renal diseases, rheumatoid arthritis, or systemic lupus erythematosus. We included in the analysis 32 subjects who reported thyroid disease and 22 subjects who reported anemia (4 subjects reported both) because these diseases are often overreported. We used the statistical methods described later in this article to determine whether our results may have been affected by including subjects who reported thyroid disease or anemia.
MEASUREMENT OF EFFECT OF FATIGUE ON QUALITY OF LIFE
We measured the degree that fatigue affected the subjects' quality of life by summing the responses to the frequency of the following 5 characterizations of energy level: (1) full of pep or energy; (2) tired, worn out, used up, or exhausted; (3) woke up feeling fresh and rested; (4) fatigue has interfered with my work, family, or social life; and (5) fatigue has been one of my 3 most disabling symptoms. The first 3 of these questions came from the 4-question RAND Vitality Index7 (the fourth RAND question seemed redundant and would have required us to use a much more complex format for our questionnaire); the fourth and fifth questions were taken from Krupp et al11 and represent the effect of the fatigue on quality of life. The 3 questions about the frequency of fatigue (questions 2, 4, and 5) were given a score of 1 for "none of the time," 2 for "a little of the time," 3 for "some of the time," 4 for "a good bit of the time," 5 for "most of the time," and 6 for "all of the time." The 2 questions relating to the frequency of having high energy (questions 1 and 3) had reverse scoring (ie, from 6 to 1). Using higher scores to represent more rather than less fatigue was arbitrary and has no effect on the conclusions of the study. We refer to the measure of the effect of fatigue on quality of life as the quality of life (QOL) fatigue score. The possible range of this score was from 5 to 30.
We excluded subjects with a total score of less than 17.5 on the 5 questions, which is an average score midway between "some of the time" and "a good bit of the time." The threshold used for this study was chosen because it corresponds to the threshold level used by the Rand Vitality Index7, 12 to define fatigue. Of the 330 subjects in the registry who completed the follow-up questionnaire, 66 were excluded because they did not meet the critical level of fatigue.
Although the individual questions in this score have been assessed elsewhere,7, 11 we evaluated the reliability and validity of the score in our data set. The ceiling effect was not substantial because only 7.7% of the subjects had the highest possible score. The internal consistency or reliability of the score as measured by Cronbach  was .69, which is quite high considering that all of the subjects in the study had fatigue that limits the dispersion of the answers to the questions. The construct validity was measured by finding the correlation of the score with several patient reports that are likely to be related to the effect of fatigue on their quality of life. The correlations between the QOL fatigue score and these other variables were as follows: r = 0.75 with physical fatigue score (constructed as defined later in this article), r = 0.55 with mental fatigue score (constructed as defined later in this article), r = 0.35 with fatigue at its worse, r = 0.53 with energy level in the past month as a percentage of optimal functioning, r = 0.41 with energy level after light exercise, and r = 0.27 with energy level after mental effort. All of these correlations were statistically significant (P<.001). In our data sets that included patients who had fatigue scores below the cutoff point, the correlations were higher. was .69, which is quite high considering that all of the subjects in the study had fatigue that limits the dispersion of the answers to the questions. The construct validity was measured by finding the correlation of the score with several patient reports that are likely to be related to the effect of fatigue on their quality of life. The correlations between the QOL fatigue score and these other variables were as follows: r = 0.75 with physical fatigue score (constructed as defined later in this article), r = 0.55 with mental fatigue score (constructed as defined later in this article), r = 0.35 with fatigue at its worse, r = 0.53 with energy level in the past month as a percentage of optimal functioning, r = 0.41 with energy level after light exercise, and r = 0.27 with energy level after mental effort. All of these correlations were statistically significant (P<.001). In our data sets that included patients who had fatigue scores below the cutoff point, the correlations were higher.
RISK FACTORS FOR CHANGES IN QOL FATIGUE SCORE
The change in QOL fatigue score was the primary outcome measure. The types of risk factors evaluated for their association with a change in QOL fatigue score included the following: demographics (age, sex, marital status, income, job status, education, race, and membership in the Wisconsin Chronic Fatigue Association); lifestyle (exercise, smoking, drinking, watching television, and eating regularly); psychological (stress, depression, psychological counseling, relative with psychological problem, sleep, worry, confidence, and satisfaction); medical (chronic diseases, frequency of acute diseases, body mass index [calculated as weight in kilograms divided by the square of height in meters], and somatoform symptoms); and characteristics of fatigue (duration of baseline QOL fatigue score, physical fatigue, and mental fatigue; type of onset; duration of fatigue after exercise; previous changes in 6 months; and factors that affect the level of fatigue). For subjects who reported that they had a relative with a psychological problem, we did not divide the subjects on the basis of the relationship of the person with the problem or the type of psychological problem reported. To obtain an indication of sleep apnea, we asked subjects whether their spouse or partner noticed that their breathing stopped during the night.
Several scales were constructed in the same way as the QOL fatigue score, ie, by summing the frequency scores for all the symptoms in the scale. These scales included a depression scale made of 14 questions from the Zung scale,13 a stress score made of 13 questions from the Social Readjustment Rating scale,14 a score for physical fatigue made of 3 questions from the Profile of Fatigue-Related Symptoms scale,15 and a score for neurocognitive problems associated with fatigue made of 7 questions from the Fatigue Impact scale.16
We also constructed 2 scales for somatoform symptoms, those symptoms that cannot be determined to have an organic basis. One scale indicated the number of somatoform symptoms used to define CFS17 that are present at least a good bit of the time. These include sore throat, tender cervical or axillary lymph nodes, muscle aches, joint pain, and headaches. A related scale counted the number of minor CFS features. This scale included all of the somatoform symptoms used to define CFS plus unrefreshing sleep, postexertional malaise over 24 hours, inability to concentrate, and sudden onset of the fatigue after an infectious illness. A second scale for somatoform symptoms, referred to as "other somatoform symptoms," included a list of common somatoform symptoms that were not included in the most recent definition of CFS: backache, indigestion, diarrhea, constipation, other stomach or intestinal discomfort, mild fever or chills, unexplained muscle weakness, and dizziness.
Other psychological characteristics (worry, self-confidence, and perfectionism) were measured with single questions about how the subject believed that she or he compared with most other people. We also asked the subjects about how satisfied or pleased they had been with their personal life during the past month.
In addition to examining potential risk factors measured at baseline, we also examined whether changes in certain risk factors from baseline to follow-up were associated with changes in fatigue. The risk factors included in this analysis were depression, sleep disturbances, and somatoform symptoms.
STATISTICAL METHODS
The outcome measure for this study was improvement in fatigue score; a negative improvement score indicated worsening. An alternative outcome that has been used in some previous studies is the final fatigue score. The disadvantage of this latter outcome measure is that the best predictor of the final fatigue score was the fatigue score at baseline. In our data, the correlation between the baseline and final fatigue scores was 0.61. Therefore, risk factors for final fatigue score are primarily risk factors for fatigue score at baseline and not necessarily risk factors for changes in fatigue score.
We tested the association between individual patient characteristics or changes in patient characteristics with an improvement in QOL fatigue score using Pearson correlations or t tests. Linear regression analysis was used to evaluate which combination of risk factors had a statistically significant independent association with improvement. By including a term for interaction in the regression analysis, we were able to evaluate whether the association between a risk factor and improvement depended on the source of the subjects, the presence of anemia or thyroid disease, or depression level at baseline.
Fatigue outcome was also categorized to make the results more intuitive. Because there were 5 questions used in the QOL fatigue score, we considered an improvement of 6 or more (ie, subjects improved by an average of more than 1 category on each question) as substantial. Subjects whose QOL fatigue score stayed the same or got worse during the 2-year follow-up were classified as not improved.
RESULTS
There were 330 subjects in the registry. Of those, 290 returned a follow-up questionnaire. Thirty-five were excluded because they did not meet the cutoff for severity of fatigue and another 56 subjects were excluded because they reported one of the severe chronic diseases listed earlier in this article. The range in follow-up for the subjects in the study was from 1.75 years to 2.9 years, with a median follow-up of 2.1 years, a 10th percentile follow-up of 1.99 years, and a 90th percentile follow-up of 2.35 years. The median QOL fatigue score was 25 (range, 18-30). The average improvement in fatigue score was 1.93 (range, -10 to 15), a 10th percentile of -2, and a 90th percentile of 7. The correlation between the length of follow-up and change in fatigue level was 0.14, which was statistically significant (P = .04).
The demographic and lifestyle characteristics of the subjects are given in Table 1 according to the source of the subjects. Of the clinic patients, 11 were from a clinic for internal medicine, 12 were from 2 clinics in which the physician practiced complementary medicine, 5 were from 2 academic family practice clinics, and 54 were from 12 private practices staffed by family physicians.
|
|

|
|
Table 1. Demographic Characteristics of Subjects*
|
|
|
Most subjects were women (87%) and were white (91%). Most were between the ages of 30 and 55 years, but 13% were older than 55 years, and 8% were between 18 and 29 years. About half were married. In general, they were well educated: 48% were college graduates. A high percentage of the subjects (27%) had a family income of less than $20,000. Although 92% of the subjects had been treated by a physician for fatigue, only 52% were being treated at the time they completed the follow-up questionnaire. A similar percentage of patients who reported thyroid disease or anemia were recruited from the medical clinics and the 2 other groups (28% vs 23%).
The relationships between the subjects' characteristics and improvement in fatigue are given in Table 2. Characteristics found to be associated with less improvement in the univariate analysis were unemployment (P = .04), more somatoform symptoms that are not used to define CFS (P = .005), frequent awakening during the night (P = .04), and having a biological relative with a psychological problem (P = .01). There was no association between change in level of fatigue with many other measures of reported health or psychological characteristics.
|
|

|
|
Table 2. Correlations Between Characteristics of the Subjects and Improvement in Fatigue
|
|
|
The association of improvement with fatigue characteristics at baseline is given in Table 3. The only statistically significant associations were with the score for neurocognitive symptoms and the frequency with which the subject reported not thinking clearly. This latter variable was used as part of the neurocognitive score.
|
|

|
|
Table 3. Correlations of Fatigue Characteristics With Fatigue Improvement
|
|
|
Table 4 gives the results of the regression analysis to identify subject characteristics that have an independent association with fatigue improvement. With one exception, all of the predictor variables included in the equation were patient characteristics measured at baseline. The one exception was length of follow-up, which was included so that the results would not be influenced by the slightly greater improvement of the subjects who were followed for the longest period. The patient characteristics independently associated with greater improvement were greater initial levels of fatigue (P<.001), clear thinking more of the time (P<.001), fewer somatoform symptoms not used to define CFS (P = .003), less frequent nighttime awakening (P = .005), currently married (P = .05), and fewer hours of sleep (P = .05).
|
|

|
|
Table 4. Risk Factors Independently Related to Worse Prognosis
|
|
|
The level of statistical significance for baseline fatigue was much greater in the regression analysis than in univariate correlation analysis. This is because people with high levels of fatigue were less likely to have few somatoform symptoms or clear thinking. In the regression equation the effect of baseline fatigue was adjusted for these 2 variables. The interpretation of the regression equation is that persons with high levels of fatigue are likely to have a greater improvement in fatigue than persons who have lower levels of fatigue but who are the same with respect to clear thinking and the number of somatoform symptoms.
Other variables that had statistically significant correlations with fatigue improvement were not statistically significant in the regression analysis because they were confounded with these variables. These variables were not having a job and having a relative with a psychological disorder.
Using statistical tests for interaction, we found that the relationship between change in fatigue and the prognostic factors did not depend on whether the subjects reported thyroid disease or anemia or received psychological counseling prior to the onset of the fatigue. The association between clear thinking and improvement was significantly stronger for members in the Wisconsin Chronic Fatigue Association than for other subjects.
As a simple indicator of fatigue prognosis we used the combination of the 2 best predictors: the frequency of clear thinking and the number of 8 somatoform symptoms not used to define CFS that occurred at least a good bit of the time. Subjects with the best prognosis had no somatoform symptoms and an ability to think clearly most of the time (ie, inability to think clearly less than most of the time). Of the 29 subjects in this category, 8 (28%) improved substantially and 7 (24%) stayed the same or deteriorated. Subjects with the worst prognosis had more than 2 somatoform symptoms and had trouble thinking clearly all of the time. Of the 29 subjects in this category, only 1 (3%) improved substantially and 18 (62%) stayed the same or deteriorated. The difference between the best and worst prognostic groups was statistically significant (P = .01) for both the percentages that improved substantially and the percentages that did not improve.
Correlations of changes in fatigue with changes in risk factors measured at baseline and follow-up are presented in Table 5. The change in fatigue was strongly linked with changes in other patient symptoms, especially clear thinking, depression, muscle aches, joint pain, and trouble falling asleep. Changes in all of these factors except joint pain (which was highly correlated with muscle aches) were also statistically significant in the regression analysis that identified changes in factors that were independently associated with changes in fatigue.
|
|

|
|
Table 5. Correlation of Changes in Risk Factors With Changes in Fatigue
|
|
|
The association between the 4 terms in the regression equation and change in fatigue was generally not affected by membership in the CFS support group, reported anemia or thyroid disease, or psychological counseling prior to the onset of fatigue. The only exception was that the association between changes in muscle aches and changes in fatigue was significantly stronger for the 166 subjects who did not have psychological counseling prior to their fatigue than for the 33 subjects who did.
We also evaluated whether the association between changes in depression level and changes in fatigue were stronger for subjects who were more depressed at baseline. To the contrary, we found that the association was stronger for those who were less depressed, but the effect of initial levels of depression was not statistically significant.
COMMENT
We evaluated the association of fatigue improvement with risk factors measured at baseline and changes in these risk factors. Because many risk factors were evaluated in this study, it was possible to compare their relative strengths. The most important baseline factors that predicted greater fatigue improvement were clear thinking, fewer somatoform symptoms not used to define CFS, infrequent nighttime awakening, fewer hours sleeping, and being married. Patient symptoms that changed concomitantly with changes in fatigue included clear thinking, depression, muscle aches, joint pain, and trouble falling asleep.
Most risk factors examined in previous studies, with the exception of specific psychological scales, were examined in this study. Many of our results agreed with those of others. As in other studies, we found a significant association of a worse prognosis with more somatoform symptoms18 and no significant association of prognosis with exercise,19 current depression,6, 8, 19 education,6, 19 age at onset,20 or psychological impairment.21 On the other hand, some of our results differed from those in previous studies. Prognostic factors from previous studies that were not found to be statistically significant in this study included the attribution of fatigue to a physical illness,21-23 race,7 use of alcohol, and belonging to a self-help organization.22 The previous finding most in conflict with ours was a worse prognosis for patients who had a greater severity of fatigue at baseline.18, 21 The reason for this difference between our study and previous studies was that the other studies used fatigue cure as the outcome measure. Although greater initial levels of fatigue decreased the chance of complete recovery, it was related to the amount of fatigue improvement. The definition of fatigue improvement may also account for discrepancies between our study and others that evaluated risk factors strongly associated with levels of baseline fatigue, eg, use of alcohol and the attribution of fatigue to a physical illness.
Other findings in our study did not have a comparison in the literature. We are not aware of previous studies that tested stress as a prognostic factor, that divided somatoform symptoms into those that are used in the definition of CFS and those that are not, or that tested an association of changes in fatigue with changes in other factors.
Because of the large number of variables examined for an association with change in fatigue level, some of the associations may have been statistically significant by chance alone. We did not use statistical methods to take into account the number of tests performed because these methods may obscure important associations.24 Because results from our study need to be confirmed with other samples even if we had adjusted for multiple comparisons, there is little risk in reporting all results that are statistically significant (P<.05).
The findings in this study may help with the understanding of fatigue. Patients with no somatoform symptoms not considered part of CFS, less difficulty with clear thinking, or less frequent nighttime awakening may have types of fatigue that are more likely to resolve than other patients. The support of a spouse may help with the management of fatigue. The change in depression score associated with changes in fatigue could be interpreted to mean that depression was a cause of the fatigue. It seems reasonable, however, that high levels of depression would be a more important cause of fatigue than low levels of depression. Therefore, a reduction in high levels of depression should be associated with a greater improvement than a reduction in low levels of fatigue. Because we did not find this difference in association, it is possible that depression covaries with fatigue even though it is not a direct cause of the fatigue.
Our findings that changes in fatigue occur together with changes in clear thinking, depression, muscle aches, and joint pain are consistent with theories that all of these conditions have a similar underlying cause. As the underlying problem improves, both fatigue and these other symptoms will improve. Although it has been suggested that this underlying cause is a constitutional disturbance such as in neurotransmitters or in the hypothalamic pituitary axis,25-27 little progress has been made in finding the specific disturbance linking these conditions. It may be revealing that the association between changes in muscle aches and changes in fatigue was significantly stronger for persons who did not have psychological counseling prior to their fatigue compared with persons who did. This result suggests that muscle aches are more closely linked to nonpsychological causes of fatigue than to psychological causes.
AUTHOR INFORMATION
Accepted for publication May 2, 1998.
This study was funded in part by a grant from the American Academy of Family Physicians, Kansas City, Mo.
We thank the Wisconsin Chronic Fatigue Syndrome Association, Madison, the Family Health Plan, Milwaukee, Wis, the Waisbren Clinic, Milwaukee, and the Wisconsin Research Network, Madison, for help with recruiting patients for the study; and Teri Wermager for help with managing patient recruitment.
Reprints: Arthur J. Hartz, MD, PhD, Department of Family Medicine University of Iowa College of Medicine, 01292-D PFP, 200 Hawkins Dr, Iowa City, IA 52242-1097.
From the Department of Family Medicine, University of Iowa College of Medicine, Iowa City (Dr Hartz and Ms Bentler); the Medical College of Wisconsin, Department of Family and Community Medicine, Milwaukee (Drs Kuhn and London); and the George Washington School of Public Health, Department of Epidemiology, Washington, DC (Dr Levine).
REFERENCES
 |  |
1. Nelson E, Kirk J, McHugo G, Douglass R, Ohler J, Wasson J, Zubkoff M. Chief complaint fatigue: a longitudinal study from the patient's perspective. Fam Pract Res J. 1987;6:175-188.
PUBMED
2. Wilson A, Hickie I, Lloyd A, Wakefield D. The treatment of chronic fatigue syndrome: science and speculation. Am J Med. 1994;96:544-549.
FULL TEXT
|
ISI
| PUBMED
3. Lloyd AR, Brockman A, Hickie C, Dwyer J, Wakefield D. Immunologic and psychologic therapy for patients with chronic fatigue syndrome: a double-blind, placebo-controlled trial. Am J Med. 1993;94:197-203.
FULL TEXT
|
ISI
| PUBMED
4. Blondel-Hill E, Shafran SD. Treatment of the chronic fatigue syndrome: a review and practical guide. Drugs. 1993;46:639-651.
ISI
| PUBMED
5. Lewis G, Wessely S. The epidemiology of fatigue: more questions than answers. J Epidemiol Community Health. 1992;46:92-97.
FREE FULL TEXT
6. Cathebras PJ, Robbins JM, Kirmayer LJ. Fatigue in primary care: prevalence, psychiatric comorbidity, illness behavior, and outcome. J Gen Intern Med. 1992;7:276-286.
ISI
| PUBMED
7. Valdini AF, Steinhardt S, Valicenti J, Jaffe A. A one-year follow-up of fatigued patients. J Fam Pract. 1988;26:33-38.
ISI
| PUBMED
8. Clark MR, Katon W, Russo J, Kith P, Sintay M, Buchwald D. Chronic fatigue: risk factors for symptom persistence in a 2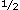 -year follow-up study. Am J Med. 1995;98:187-195.
FULL TEXT
|
ISI
| PUBMED
9. Murphy KA, Cornish RD. Prediction of chronicity in acute low back pain. Arch Phys Med Rehabil. 1984;65:334-337.
ISI
| PUBMED
10. Crook J, Tunks E, Kalaher S, Roberts J. Coping with persistent pain: a comparison of persistent pain sufferers in a specialty pain clinic and in a family practice clinic. Pain. 1988;34:175-184.
FULL TEXT
|
ISI
| PUBMED
11. Krupp LB, La Rocca NG, Muir-Nash J, Steinberg AD. The Fatigue Severity Scale: application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol. 1989;46:1121-1123.
FREE FULL TEXT
12. Valdini AF, Steinhardt SI, Jaffe AS. Demographic correlates of fatigue in a university family health centre. Fam Pract. 1987;4:103-107.
FREE FULL TEXT
13. Zung WWK. A self-rating depression scale. Arch Gen Psych. 1965;12:63-70.
14. Holmes TH, Rahe RH. The Social Readjustment Rating scale. J Psychosom Res. 1967;11:213-218.
FULL TEXT
|
ISI
| PUBMED
15. Ray C, Weir WRC, Phillips S, Cullen S. Development of a measure of symptoms in chronic fatigue syndrome: the Profile of Fatigue-Related Symptoms (PFRS). Psych Health. 1992;7:27-43.
FULL TEXT
16. Fisk JD, Ritvo PG, Ross L, Haase DA, Marrie TJ, Schlech WF. Measuring the functional impact of fatigue: initial validation of the Fatigue Impact scale. Clin Infect Dis. 1994;18(suppl 1):S79-S83.
17. Fukuda K, Straus SE, Hickie I, et al. The chronic fatigue syndrome: a comprehensive approach to its definition and study. Ann Intern Med. 1994;121:953-959.
FREE FULL TEXT
18. Bonner D, Ron M, Chalder T, Butler S, Wessely S. Chronic fatigue syndrome: a follow-up study. J Neurol Psych. 1994;57:617-621.
FULL TEXT
19. Kroenke K, Wood DR, Mangelsdorff D, Meier NJ, Powell JB. Chronic fatigue in primary care prevalence, patient characteristics, and outcome. JAMA. 1988;260:929-934.
FREE FULL TEXT
20. Wilson A, Hickie I, Lloyd A, et al. Longitudinal study of outcome of chronic fatigue syndrome. BMJ. 1994;308:756-759.
FREE FULL TEXT
21. Vercoulen JHMM, Swanink CMA, Fennis JFM, Galama JMD, van der Meer JWM, Bleijenberg G. Prognosis in chronic fatigue syndrome: a prospective study on the natural course. J Neurol Neurosurg Psychiatry. 1996;60:489-494.
FREE FULL TEXT
22. Sharpe M, Hawton K, Seagroatt V, Pasvol G. Follow up of patients presenting with fatigue to an infectious diseases clinic. BMJ. 1992;305:147-152.
23. Wilson A, Hickie I, Lloyd A, Wakefield D. The treatment of chronic fatigue syndrome: science and speculation. Am J Med. 1994;96:544-550.
24. Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1:43-46.
PUBMED
25. Straus SE. History of chronic fatigue syndrome. Rev Infect Dis. 1991;13:52-57.
ISI
| PUBMED
26. Gruber AJ, Hudson JL, Pope HG. The management of disorders on the interface of psychiatry and medicine: fibromyalgia, chronic fatigue syndrome, migraine, irritable bowel syndrome, atypical facial pain and premenstrual dysphoria disorder. Psychiatr Clin North Am. 1996;19:351-369.
FULL TEXT
|
ISI
| PUBMED
27. Klimas NG, Morgan R, VanRiel F, Fletcher MA. Observations regarding use of an antidepressant, fluoxetine, in chronic fatigue syndrome. In: Goodnick PJ, Klimas NG, eds. Chronic Fatigue and Related Immune Deficiency Syndromes: Progress in Psychiatry. No 40. Washington, DC: American Psychiatric Press Inc; 1993:95-108. -year follow-up study. Am J Med. 1995;98:187-195.
FULL TEXT
|
ISI
| PUBMED
9. Murphy KA, Cornish RD. Prediction of chronicity in acute low back pain. Arch Phys Med Rehabil. 1984;65:334-337.
ISI
| PUBMED
10. Crook J, Tunks E, Kalaher S, Roberts J. Coping with persistent pain: a comparison of persistent pain sufferers in a specialty pain clinic and in a family practice clinic. Pain. 1988;34:175-184.
FULL TEXT
|
ISI
| PUBMED
11. Krupp LB, La Rocca NG, Muir-Nash J, Steinberg AD. The Fatigue Severity Scale: application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol. 1989;46:1121-1123.
FREE FULL TEXT
12. Valdini AF, Steinhardt SI, Jaffe AS. Demographic correlates of fatigue in a university family health centre. Fam Pract. 1987;4:103-107.
FREE FULL TEXT
13. Zung WWK. A self-rating depression scale. Arch Gen Psych. 1965;12:63-70.
14. Holmes TH, Rahe RH. The Social Readjustment Rating scale. J Psychosom Res. 1967;11:213-218.
FULL TEXT
|
ISI
| PUBMED
15. Ray C, Weir WRC, Phillips S, Cullen S. Development of a measure of symptoms in chronic fatigue syndrome: the Profile of Fatigue-Related Symptoms (PFRS). Psych Health. 1992;7:27-43.
FULL TEXT
16. Fisk JD, Ritvo PG, Ross L, Haase DA, Marrie TJ, Schlech WF. Measuring the functional impact of fatigue: initial validation of the Fatigue Impact scale. Clin Infect Dis. 1994;18(suppl 1):S79-S83.
17. Fukuda K, Straus SE, Hickie I, et al. The chronic fatigue syndrome: a comprehensive approach to its definition and study. Ann Intern Med. 1994;121:953-959.
FREE FULL TEXT
18. Bonner D, Ron M, Chalder T, Butler S, Wessely S. Chronic fatigue syndrome: a follow-up study. J Neurol Psych. 1994;57:617-621.
FULL TEXT
19. Kroenke K, Wood DR, Mangelsdorff D, Meier NJ, Powell JB. Chronic fatigue in primary care prevalence, patient characteristics, and outcome. JAMA. 1988;260:929-934.
FREE FULL TEXT
20. Wilson A, Hickie I, Lloyd A, et al. Longitudinal study of outcome of chronic fatigue syndrome. BMJ. 1994;308:756-759.
FREE FULL TEXT
21. Vercoulen JHMM, Swanink CMA, Fennis JFM, Galama JMD, van der Meer JWM, Bleijenberg G. Prognosis in chronic fatigue syndrome: a prospective study on the natural course. J Neurol Neurosurg Psychiatry. 1996;60:489-494.
FREE FULL TEXT
22. Sharpe M, Hawton K, Seagroatt V, Pasvol G. Follow up of patients presenting with fatigue to an infectious diseases clinic. BMJ. 1992;305:147-152.
23. Wilson A, Hickie I, Lloyd A, Wakefield D. The treatment of chronic fatigue syndrome: science and speculation. Am J Med. 1994;96:544-550.
24. Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1:43-46.
PUBMED
25. Straus SE. History of chronic fatigue syndrome. Rev Infect Dis. 1991;13:52-57.
ISI
| PUBMED
26. Gruber AJ, Hudson JL, Pope HG. The management of disorders on the interface of psychiatry and medicine: fibromyalgia, chronic fatigue syndrome, migraine, irritable bowel syndrome, atypical facial pain and premenstrual dysphoria disorder. Psychiatr Clin North Am. 1996;19:351-369.
FULL TEXT
|
ISI
| PUBMED
27. Klimas NG, Morgan R, VanRiel F, Fletcher MA. Observations regarding use of an antidepressant, fluoxetine, in chronic fatigue syndrome. In: Goodnick PJ, Klimas NG, eds. Chronic Fatigue and Related Immune Deficiency Syndromes: Progress in Psychiatry. No 40. Washington, DC: American Psychiatric Press Inc; 1993:95-108.
RELATED ARTICLE
The Archives of Family Medicine Continuing Medical Education Program
Arch Fam Med. 1999;8(6):543-545.
FULL TEXT
THIS ARTICLE HAS BEEN CITED BY OTHER ARTICLES
Prospective Study of the Prognosis of Unexplained Chronic Fatigue in a Clinic-Based Cohort
Schmaling et al.
Psychosom. Med. 2003;65:1047-1054.
ABSTRACT
| FULL TEXT
|