 |
 |

New Antiepileptic Drugs
Into the New Millennium
William O. Tatum, IV, DO;
Rupert Galvez, BA;
Selim Benbadis, MD;
Enrique Carrazana, MD
Arch Fam Med. 2000;9:1135-1141.
ABSTRACT
There has been an explosion of new antiepileptic drug availability for physicians to treat patients with recurrent seizures. Principal antiepileptic drugs consisted of 6 key agents for both generalized and partial epilepsy for nearly 8 decades. Since 1993, the availability of newer "second-generation" agents has nearly doubled the armamentarium available for the 2.5 million patients who have recurrent seizures. This new influx of medications has flooded the medical and lay community with choices never before appreciated. The promise of improved tolerability with different safety and efficacy profiles has been exciting for all involved in epilepsy management. While most of the newer agents have been approved for adjunctive use in medically refractory partial epilepsy with recurrent complex partial and secondarily generalized seizures, efficacy is expanding to include generalized epilepsy and children for some agents.
INTRODUCTION
Seizures are one of the most common neurologic disorders of brain function. Epilepsy is the condition of chronic recurrent epileptic seizures. Epilepsy is frequently managed by a neurologist in conjunction with a primary care physician. Approximately 3% of Americans will be diagnosed with epilepsy at some point in their lives.1 For these individuals, antiepileptic drugs (AEDs) will become the cornerstone of treatment to the traumatic obstacle created by seizures that limit living a normal life. Since the inception of traditional AEDs in 1912 when phenobarbital (Luminal; Sterling Winthrop Inc, New York, NY) initially became available, phenytoin (PHT) (Dilantin; Parke-Davis, Morris Plains, NJ), primidone (Mysoline; Wyeth-Ayerst Laboratories, Philadelphia, Pa), ethosuximide (Zarontin; Parke-Davis), carbamazepine (Tegretol; Ciba-Geigy Corp, Summit, NJ), and valproic acid (Depakene; Abbott Laboratories, North Chicago, Ill) (divalproex sodium [Depakote; Abbott Laboratories]) were released within the next 70 years. The management of epilepsy has remained confined to one or a combination of these standard AEDs. Since 1993, a 15-year hiatus of AED availability has been interrupted by the release of newer AEDs, including felbamate (Felbatol; Carter-Wallace, Cranbury, NJ), gabapentin (Neurontin; Parke-Davis), lamotrigene (LTG) (Lamictal; Glaxo Wellcome Inc, Research Triangle Park, NC), topiramate (TPM) (Topamax; Ortho-McNeil Pharmaceutical, Raritan, NJ), and tiagabine hydrochloride (TGB) (Gabitril; Abbott Laboratories) during the last 5 years. The newest AEDs include oxcarbazepine (OXC) (Trileptal; Novartis Pharmaceuticals, East Hanover, NJ), levetiracetam (Keppra; UCB Pharmaceuticals Inc, Smyrna, Ga), and zonisamide (ZNS) (Zonegran; Elan Pharmaceuticals, South San Francisco, Calif), which further the availability of additional AEDs. Furthermore, new formulations and chemical alterations of traditional AEDs have become available, thus increasing the potential for new routes of administration, improved safety profiles, and less frequent dosing schedules. The new "second-generation" AEDs have shown different efficacy and tolerability profiles that set them apart from the standard and older "first-generation" counterparts. The following is a chronological review of the new AEDs released for patients with epilepsy.
THE NEW ANTIEPILEPTIC DRUGS
Since 1993, with the release of felbamate heralding the appearance of the new or second-generation AEDs, 5 additional medications have been released for patients with epilepsy. This was the first group of AEDs to offer an alternative to the old or standard first-generation AEDs most neurologists and primary care physicians alike have the greatest experience utilizing.
Felbamate
Felbamate (Felbatol) was released in 1993 for adjunctive use as well as monotherapy for patients with partial seizures and for patients older than age 2 years with Lennox-Gastaut syndrome (LGS) following completion of controlled studies with novel designs.2 Its mechanism displays a broad profile and includes effects on the Na+, Ca++, and NMDA receptors. It was similarly found to have a clinical broad spectrum of activity in both partial and generalized seizures with additional efficacy suggested in juvenile myoclonic epilepsy and infantile spasms. Improved alertness was noted with felbamate during the Lennox-Gastaut trial.3 In August 1994, withdrawal of US patients was recommended by Carter-Wallace in conjunction with the Food and Drug Administration (FDA), Washington, DC, following initial reports of fatal aplastic anemia and subsequently, hepatic failure. Aplastic anemia has occurred with a risk of 1 in 2000 to 5000 patients, and hepatic failure, in approximately 1 in 30 000 receiving the drug. Therefore, a thorough signed informed consent form and performance of frequent complete blood cell counts and liver function tests during the course of treatment are recommended by the pharmaceutical company. Felbamate has largely been replaced by alternative AEDs though may still be used in serious cases of epilepsy when the risks of uncontrolled epilepsy seem to outweigh the potential risks from the drug.
Gabapentin
Gabapentin is a 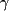 -aminobutyric acid (GABA) analog marketed under the trade name Neurontin by Parke-Davis since 1994. It is effective as adjunctive therapy for adults with partial and secondarily generalized seizures.4 Trials of monotherapy have had mixed results.5-6 Using the doses studied, it has not proven effective for generalized seizure types and absence epilepsy. Off-label use of gabapentin is increasingly being recognized as a useful first-line agent in the treatment of neuropathic pain7 and other pain syndromes and also shows promise in treating psychiatric conditions.8 Mechanistically, a novel binding site in the brain has been demonstrated at a subunit of the Ca++ channel. The most frequent adverse effects include somnolence, dizziness, ataxia, fatigue, and nystagmus. Other effects include nausea, weight gain, tremor, and diplopia. Serious adverse effects have not been noted in more than 2 million people receiving gabapentin worldwide. -aminobutyric acid (GABA) analog marketed under the trade name Neurontin by Parke-Davis since 1994. It is effective as adjunctive therapy for adults with partial and secondarily generalized seizures.4 Trials of monotherapy have had mixed results.5-6 Using the doses studied, it has not proven effective for generalized seizure types and absence epilepsy. Off-label use of gabapentin is increasingly being recognized as a useful first-line agent in the treatment of neuropathic pain7 and other pain syndromes and also shows promise in treating psychiatric conditions.8 Mechanistically, a novel binding site in the brain has been demonstrated at a subunit of the Ca++ channel. The most frequent adverse effects include somnolence, dizziness, ataxia, fatigue, and nystagmus. Other effects include nausea, weight gain, tremor, and diplopia. Serious adverse effects have not been noted in more than 2 million people receiving gabapentin worldwide.
The pharmacokinetics make this agent clinically attractive. A dose-dependent bioavailability limits absorption and peaks 1 to 3 hours after oral administration. The drug is not protein bound and exhibits no appreciable hepatic metabolism. Gabapentin is excreted based on renal function, and dose adjustments are required based on creatinine clearance. The terminal half-life is 5 to 7 hours but is longer with impaired renal function. Dosing is usually divided into 3 daily doses, although in patients receiving hemodialysis, a single dose given after each dialysis is recommended. In epilepsy management, doses of 3600 to 4800 mg/d have been used with both tolerability and efficacy beyond the maximum FDA-approved dose of 1800 mg/d. The safety and tolerability, as well as the pharmacokinetics, make gabapentin a favorable AED in patients in need of quick titration, multiple AED intolerances, those receiving multiple drugs with the potential for interaction (ie, elderly patients), patients with hepatic disease (ie, porphyria), and patients with prior drug overdose.
Lamotrigene
Glaxo Wellcome Inc gained approval for marketing Lamictal in 1995 initially for adjunctive treatment of adults with partial seizures. Placebo-controlled trials initially demonstrated efficacy in refractory complex partial and secondarily generalized seizures.9-10 It has since demonstrated efficacy in children older than age 2 years with LGS11 and in monotherapy after converting from an initiating AED.12 Increasing evidence has suggested efficacy in generalized seizures, including absence seizures.13 Efficacy in neuropathic pain14 and bipolar disorder15 (especially the depressive phase) has been shown. Similar to PHT and carbamazepine, LTG acts at the Na+ channel, but may also modulate Ca++ channels or have other mechanisms to explain its broad spectrum of activity against multiple seizure types. The most common adverse effects in monotherapy include headache, asthenia, rash, nausea, and dizziness. Somnolence was notably absent in patients given monotherapy. Additionally, in some patients, an "alerting" response has been a favorable observation with LTG. Perhaps the most well-recognized idiosyncratic adverse effect, similar to other older AEDs, is rash, which occurs in approximately 10% of patients. Infrequently (0.3% in adults), the rash can be serious and may progress to life-threatening Stevens-Johnson syndrome or toxic epidermolysis necrosis and is so indicated in the package insert. This adverse effect may be facilitated in children (1% of pediatric patients), coadministration with valproate sodium, or a faster titration.16
Lamotrigine is almost completely absorbed after oral intake and is metabolized primarily through glucuronidation in the liver. The elimination half-life is 25 hours in monotherapy but is reduced to 14 hours with drugs inducing hepatic metabolism (ie, carbamazepine) and prolonged to 60 hours with valproate. Therefore, higher doses may be needed with enzyme-inducing drugs, and lower doses are required for use with valproate. Doses of 300 to 500 mg/d initially with bimonthly increases in increments of 50 mg/d are recommended. With valproate, it is advised the initial dosing begin with 25 mg given every other day, with bimonthly increases, until reaching 150 mg/d. Lamotrigene may become useful owing to its broad spectrum of activity in generalized epilepsies such as juvenile myoclonic epilepsy and for newly diagnosed and elderly patients with epilepsy due to the low incidence of sedation.
Topiramate
Topamax is the registered trade name of TPM marketed by Ortho-McNeil Pharmaceuticals and initially became available in 1997 for adult patients with refractory partial seizures. It has demonstrated a broad spectrum of activity clinically with efficacy demonstrated as adjunctive therapy in adult and pediatric patients with refractory partial-onset seizures,17-19 including monotherapy.20 Generalized tonicoclonic seizures in adults and children older than age 2 years also respond to TPM,21 in addition to showing reduction of seizures and drop attacks in a controlled trial with TPM in patients with LGS.22 Topiramate uses 3 main mechanisms of antiepileptic action, including Na+ channel blockade, potentiation of GABA-mediated inhibition, and antagonism of a subtype of NMDA-activated neuronal excitation that leads to seizures. Weak carbonic hydrase inhibition is also present. Preliminary efficacy in chronic bipolar disorder23 and pain control of migraine24 seems promising for off-label use. The most frequent adverse effects of TPM are somnolence, dizziness, confusion, ataxia, and diplopia, and the effects frequently seem to be titration related.17-18,25 Weight loss has been noted and typically seen during the first 3 months. Nephrolithiasis occurred in 1.5% of patients, and paresthesias likely reflect the carbonic anhydrase inhibition. No serious systemic idiosyncratic adverse effects have been recognized so far. Topiramate is rapidly absorbed with substantial bioavailability 2 hours after oral dosing. Topiramate has linear pharmacokinetics and is not extensively metabolized in the liver. Approximately 50% of the dose is excreted by the kidney, requiring reduction in renal failure. About 17% of protein binding to TPM occurs with minimal drug-drug interactions. Topiramate reduces the efficacy of oral contraceptives but has no clinically serious effect on AEDs. Phenytoin clearance decreased in some patients, leading to higher PHT levels. Alternatively, other enzyme-inducing AEDs reduce the plasma concentrations of TPM, leading to higher TPM levels on withdrawal of the concomitant agent. A slow titration of 25 to 50 mg per week reaching 200 to 400 mg per day may reduce the risk of adverse effects.
Tiagabine
Gabatril was approved by the FDA in 1997 for marketing by Abbott Laboratories. It was designed to inhibit the reuptake of GABA into the central nervous system, a novel mechanism that would augment inhibition of seizures. It is indicated as adjunctive therapy in patients with refractory partial-onset seizures and has been proven safe and effective in controlled clinical trials.26-27 Monotherapy may be effective for partial seizures.28 The most common adverse effects include dizziness, asthenia, somnolence, nausea, and nervousness. No serious systemic or serious idiosyncratic adverse events have been noted thus far. Tiagabine increases synaptic GABA as it was designed. Bioavailability is 89%, and protein binding is 96% with more rapid absorption occurring without food. As such, food is recommended when receiving TGB. Hepatic metabolism is by the P450 family of enzymes and is linear. The elimination half-life is 5 to 8 hours and shortened to 2 to 3 hours with enzyme-inducing AEDs. Clearance is increased, reducing the serum concentrations of TGB with enzyme-inducing AEDs. Tiagabine does not affect the serum concentration of other drugs, though the effect on oral contraceptives is unestablished at higher doses. The initial starting doses are 4 mg per week with increases of 4 to 8 mg per week, until taking 32 to 64 mg per day, 3 times daily.
THE NEWEST ANTIEPILEPTIC DRUGS
Despite the rapidly expanding AED pharmacopeia, approximately a dozen future drugs will be seeking approval or are currently in clinical trials. The following are the newest AEDs that have become available for patients this year.
Levetiracetam
Levetiracetam, registered as Keppra by UCB Pharmaceuticals Inc, quickly received approval 10 months after its new drug application, on December 1, 1999, for adjunctive use in partial-onset seizures in adults with epilepsy and will soon be available for use. The efficacy of levetiracetam has been established in 3 controlled clinical studies.29-30 The precise mechanism of action is unknown although it seems chemically unique.31 Absorption is rapid, and the metabolism is linear and not through the liver. Treatment with this drug can begin with an initial effective daily dose, achieving a steady state after just 2 days given its half-life of 6 to 8 hours. Drug-drug interactions are minimal with less than 10% of the drug protein bound. The most attractive quality of this drug is the pharmacokinetics. More than 60% is renally excreted unchanged, though elimination is renal dependent, and caution in dosing is recommended with renal insufficiency. Adverse effects with levetiracetam reported in clinical studies include somnolence, asthenia, infection, and dizziness.30 Initial treatment with 500 mg twice daily with 1000 mg increases every 2 weeks to a maximum of 3000 mg per day is recommended. Levetiracetam has a novel design, absent appreciable hepatic metabolism, no AED interactions, and efficacy that make it an attractive choice for patients with persistent partial seizures.
Oxcarbazepine
Oxcarbazepine (Trileptal) is manufactured by Novartis Pharmaceuticals. Its new drug application was deemed "approvable" in September 1999 and then received final clearance from the FDA in early 2000. Oxcarbazepine was designed to be structurally similar to carbamazepine and is indicated for use as monotherapy in adults or adjunctive therapy in patients with partial seizures who are older than age 4 years.32-33 Oxcarbazepine has been used in other countries as an adjunctive treatment in addition to monotherapy in adults. Oxcarbazepine produces its anticonvulsant effects by regulating neuronal ion flux and inhibition of the voltage-dependent Na+ channel. Oxcarbazepine is similar to carbamazepine in terms of efficacy but may have better tolerability. Somnolence, headache, nausea, rash, and diplopia are the common adverse effects associated with OXC. Hyponatremia is a systemic effect of OXC.
Oxcarbazepine is a prodrug that is rapidly absorbed and converted to its active metabolite, mono-hydroxy-carbamazepine. Its elimination is catalyzed via noninducible cytosolic reductases though it induces the P450 liver enzyme system for metabolism. It follows first-order kinetics. Drug-drug interactions are less than with carbamazepine, and protein binding is minimal. The increased tolerability of OXC over carbamazepine is most likely due to the lack of epoxide formation on metabolism. In adults, monotherapy doses typically begin at 300 to 600 mg/d, slowly titrating until reaching 900 to 2400 mg/d, 2 or 3 times daily.
Zonisamide
Zonisamide, marketed as Zonegran (Elan Pharmaceuticals Inc) and manufactured by Dainippon Pharmaceutical, Japan, has seen widespread use over the past several years in Japan. It received its drug approvable status by the FDA in March of 1998 and became available for marketing this year. Zonisamide possesses a broad spectrum of efficacy, including partial and generalized seizures.34 Moreover, it has demonstrated high efficacy in progressive myoclonic epilepsy syndromes.35 It is fully, rapidly absorbed, has a long half-life of 50 to 68 hours, and 40% to 60% protein binding. As a sulphonamide derivative, the concentration of the drug is higher in erythrocytes than in plasma. Zonisamide metabolism involves the P450 system, but excretion of the drug and its metabolites is mostly by the kidneys. Drug-drug interaction is also minimal. Zonisamide mechanisms of action include a blockade of T-type Ca++ channels, blockade of voltage-dependent Na+ channels, and inhibition of carbonic anhydrase.31 The most common adverse effects seen in double-blind, placebo-controlled studies were somnolence, ataxia, loss of appetite, confusion, fatigue, and dizziness.36 Overall, nephrolithiasis occurred in 2.6% of patients.
Zonisamide's suggested initial dosing is 100 to 200 mg with increasing doses at 2-week intervals to achieve clinical effectiveness of 400 to 600 mg in twice-daily dosing. The properties of ZNS with clinical use highlight the potential of broad-spectrum efficacy, single daily dosing, long half-life, and a unique method of action.
NEW FORMULATIONS
New formulation of traditional AEDs have become available, providing added routes and ease of administration in the treatment of patients with recurrent seizures. Two new formulations of carbamazepine in patients with complex partial seizures has the capability for twice-daily dosing with tegretol oros (Tegretol XR; Novartis Pharmaceuticals) and carbamazepine extended-release capsules (Carbitrol; Shire Richwood Inc, Florence, Ky). Valproate (Depacon; Abbott Laboratories) is now available in the intravenous (IV) form, facilitating replacement in acute situations or when oral intake is not possible. Efficacy with rapid loading in the treatment of status epilepticus has yet to be proven. Diastat (rectal diazepam; Elan Pharmaceuticals) is diazepam capable of utilization in the home setting for patients with acute repetitive (cluster) seizures that break through on maintenance AEDs.
Chemical Modifications of Traditional AEDs
Fosphenytoin sodium (Cetebyx; Parke-Davis) is a water-soluble PHT prodrug. It may be administered IV or intramuscularly. It treats the same type of seizures as PHT and has become a major breakthrough in treating status epilepticus due to a faster and safer administration. Unlike PHT, fosphenytoin does not require propylene glycol and ethanol diluents. Hence, pain, burning, cording, and phlebitis is rarely seen with fosphenytoin. Other common cardiac adverse effects, including arrhythmia and hypotension, are less frequent with fosphenytoin; however, cardiac monitoring is still recommended. A dose is expressed as PHT equivalents. Fosphenytoin infusions may be given 3 times faster at rates of 150 mg PHT equivalents per minute as opposed to 50 mg per minute for maximum PHT administration with total infusion times of approximately 7 minutes.37 Conversion of fosphenytoin to PHT requires about 8 to 15 minutes. Therapeutic levels of intramuscular fosphenytoin may be obtained in 30 minutes, and no cardiac monitoring is necessary.38 Adverse effects are similar to those observed with IV PHT, except reversible perineal pruritus and paresthesia may occur with rapid or high doses of fosphenytoin. Expense has been an obstacle to wider application.
Selection of New Agents
For most patients with epilepsy, treatment with AEDs will achieve seizure control, though despite optimal treatment, approximately 20% to 30% of patients cannot obtain successful seizure control without unacceptable adverse effects.39 Patients with primary generalized epilepsy, including absence epilepsy and idiopathic epilepsy with primary generalized tonicoclonic seizures, will respond to ethosuximide in the case of absence (though not effective for generalized tonicoclonic seizures), or to valproate in more than 80% of cases. Valproate is usually the drug of choice for generalized idiopathic epilepsies.40-41 With certain forms of primary generalized epilepsy (ie, juvenile myoclonic epilepsy), the response rates to valproate may be more than 85% to 90%. In addition, the new IV formulation of valproate may be helpful in loading for either initiation, replacement, or perioperative maintenance of effective therapy. Other newer agents with a broad spectrum of activity include felbamate, LTG, and TPM. Felbamate use has been seriously compromised owing to rare fatalities due to aplastic anemia and hepatic failure and is now only used in patients with serious epilepsy not responding to other AEDs.42 While new LTG and TPM have demonstrated efficacy in the symptomatic or secondary generalized epilepsies such as those patients with LGS,11, 16, 21-22 their efficacy in certain idiopathic primary generalized epilepsies also seems to reflect promising alternatives to valproate. With the newest AEDs, ZNS seems promising as a broad spectrum AED34 with preliminary suggestions of efficacy in progressive myoclonus epilepsies.35 Levetiracetam additionally may prove to be a broad spectrum29 agent as experience grows.30 The use of OXC may be of greater practical benefit in partial epilepsy given the possibility of carbamazepine (as well as PHT) or its metabolites exacerbating minor motor seizures.43
The treatment of patients with partial epilepsy is frequently less successful than for patients with primary generalized epilepsy. Up to 30% of patients will continue to manifest seizures despite treatment with properly selected AEDs.40-41 Carbamazepine and PHT are considered drugs of choice for patients with partial seizures.39-41 While all the newly marketed AEDs since 1993 are effective in treating patients with partial-onset seizures, most are approved for adjunctive use. Felbamate, LTG, TPM, and OXC are also approved for initial monotherapy. Pharmacokinetics and adverse effect profiles are becoming useful as selection factors for AED uses. Gabapentin and levetiracetam have negligible protein binding and hepatic metabolism, denoting minimal drug-drug interactions. Such properties may make these agents earlier considerations for elderly patients receiving multiple drug treatments. Gabapentin seems to be unusually free of adverse effects with chronic administration and may make it an ideal choice for patients with multiple AED intolerances, hepatopathies,44 or safety concerns. Lamotrigene is well-tolerated though with pharmacokinetic interactions with valproate that heighten the risk for rash. This agent may be useful when a positive psychotropic effect is desired. Topiramate seems quite potent and useful for the difficult to treat epilepsies, though like LTG and TGB, slow titration is recommended due to minimizing the potential for central nervous system adverse events. Tiagabine possesses a specific GABAergic mechanism of action that may be useful in combining "rational" AED combinations. Whether OXC will come to replace carbamazepine is doubtful given the different safety and efficacy profiles and cost issues. Higher doses of OXC obtainable may allow for greater efficacy in certain patients. Levetiracetam is unique in that the starting dose is an effective dose. No titration is required, and tolerability seems favorable despite the limited experience present at this time. Zonisamide will offer daily dosing potential not seen with any other agent but for the older agents PHT and phenobarbital, and it will provide a unique dosing profile.
Drug formulations and routes of administration using IV valproate will create new possibilities for patients in need of rapid loading for purposes of seizure control, replacement therapy, or initiation of therapy. Fosphenytoin is superior to PHT regarding safety and speed of administration.37-38 Rectal diazepam gel brings emergency medical care to the home environment by empowering caretakers of patients with uncontrolled epilepsy the chance to stop seizures earlier. Cost is always an issue and may dictate which AEDs are selected. While many new advances have been seen regarding the spectrum of activity, safety, and tolerability profiles, older agents such as PHT and carbamazepine continue to be used for patients with recurrent seizures. The treatment of seizures is evolving as more and more AEDs become available for clinical use.
CONCLUSIONS
For the 20% to 30% of patients with uncontrolled seizures, the newer AEDs bring hope and promise. For those with adverse reactions to AEDs, the newer drugs bring a new opportunity to obtain seizure control without adverse effects. Although the goal of obtaining a seizure-free status after 2 or more trials of initial AED treatment is minimal, efficacy for some and tolerability for others mean a lifetime of considerable advancement. Newer agents such as felbamate, LTG, and TPM with broad-spectrum efficacy promises greater applicability to those with generalized epilepsy and mixed seizure disorders not amenable to treatment with more traditional agents. Improved safety and tolerability profiles seen with gabapentin, LTG, TPM, and TGB may find a role in redefining the clinical approach to newly diagnosed patients with epilepsy, patients receiving multiple medications or with prior drug intolerances, and special populations, including pediatric and elderly patients.
The past decade of the brain has seen a bounty of new AEDs in addition to important new formulations and modifications of traditional drugs that improve on the safety and availability of AEDs already in use. Fosphenytoin (Cetebyx) and IV valproate (Depacon) can augment maintenance oral therapy and can potentially be used for rapid loading. Rectal diazepam (Diastat; Elan Pharmaceuticals) has further extended acute seizure management out of the hospital setting and into the home environment. Extended-release formulations of carbamazepine (Tegretol XR; Carbitrol; Shire Richwood Inc) allow for dosing twice a day to facilitate better compliance.
The new millennium will bring us yet more opportunities with AEDs such as levetiracetam, OXC, and ZNS. The need for new AEDs and new formulations remain important options for patients currently living with epilepsy. All of the new AEDs have benefits and drawbacks; however, each additional drug expands the options to offer the individual patient with seizures. With the ever-increasing pool of AEDs, it is important that the primary care physician be aware of the options for effective comanagement.
AUTHOR INFORMATION
Accepted for publication August 9, 2000.
Corresponding author: William O. Tatum IV, DO, 13801 Bruce B. Downs Blvd, Suite 401, Tampa, FL 33613 (e-mail: WOTIV{at}aol.com).
From the Department of Neurology (Dr Tatum), Epilepsy Center and the Department of Neurology and Neurosurgery (Dr Benbadis), Tampa General Hospital, University of South Florida, Tampa (Drs Tatum and Benbadis and Mr Galvez), and the Department of Neurology, Baptist Medical Center, University of Miami, Miami, Fla (Dr Carrazana).
REFERENCES
 |  |
1. Hauser WA, Annegers JF, Kurland LT. Prevalence of epilepsy in Rochester, Minnesota: 1940-1980. Epilepsia. 1991;32:429-445.
ISI
| PUBMED
2. Felbatol [package insert]. Cranbury, NJ; Carter-Wallace; 1993.
3. Felbamate Study Group in Lennox-Gastaut Syndrome. Efficacy of felbamate in childhood epileptic encephalopathy (Lennox-Gastaut syndrome). N Engl J Med. 1993;328:29-33.
FREE FULL TEXT
4. The US Gabapentin Study Group No. 5. Gabapentin as add-on therapy in refractory partial epilepsy: a double-blind, placebo-controlled, parallel-group study. Neurology. 1993;43:2292-2298.
FREE FULL TEXT
5. Bergey GK, Morris HH, Rosenfeld W, et al. Gabapentin monotherapy, I: an 8-day, double-blind, dose-controlled, multicenter study in hospitalized patients with refractory complex partial or secondarily generalized seizures. Neurology. 1997;49:739-745.
FREE FULL TEXT
6. Beydun A, Fischer J, Labar DR, et al. Gabapentin monotherapy, II: a 26-week, double-blind, dose-controlled, multicenter study of conversion from polytherapy in outpatients with refractory complex partial or secondarily generalized seizures. Neurology. 1997;49:746-752.
FREE FULL TEXT
7. Backonja M, Beydoun A, Edwards KR, et al. Gabapentin for the symptomatic treatment of painful neuropathy in patients with diabetes mellitus: a randomized controlled trial. JAMA. 1998;280:1831-1836.
FREE FULL TEXT
8. McElroy SL, Soutullo CA, Keck PE, Kmatz GE. A pilot trial of adjunctive gabapentin in the treatment of bipolar disorder. Ann Clin Psychiatry. 1997;9:99-142.
FULL TEXT
| PUBMED
9. Matsuo F, Bergen D, Faught E, Messenheimer JA, Dren AT, Rudd GD, Lineberry CG. Placebo-controlled study of the efficacy and safety of lamotrigine in patients with partial seizures. Neurology. 1993;43:2284-2291.
FREE FULL TEXT
10. Messenheimer J, Ramsay RE, Willmore LJ, et al. Lamotrigine therapy for partial seizures: a multicenter, placebo-controlled, double-blind, crossover trial. Epilepsia. 1994;35:113-121.
FULL TEXT
|
ISI
| PUBMED
11. Motte J, Trevathan E, Arvidsson JFV, Barrera MN, Mullins EL, Manasco P. The Lamictal Lennox-Gastaut Study Group: lamotrigine for generalized seizures associated with the Lennox-Gastaut syndrome. N Engl J Med. 1997;337:1807-1812.
FREE FULL TEXT
12. Gilliam F, Vazquez B, Sackellares JC, et al. An active-control trial of lamotrigine monotherapy for partial seizures. Neurology. 1998;51:1018-1025.
FREE FULL TEXT
13. Mikati MA, Holmes GL. Lamotrigine in absence and primary generalized epilepsies. J Child Neurol. 1997;12(suppl 1):S29-S37.
14. McCleane G. Lamotrigine can remove the pain associated with painful diabetic neuropathy. Eur J Neurol. 1998;5:167-173.
FULL TEXT
|
ISI
| PUBMED
15. Calabrese JR, Bowden CL, Sachs GS, et al. A double-blind placebo-controlled study of lamotrigine monotherapy in outpatients with bipolar 1 depression. J Clin Psychiatry. 1999;60:79-88.
ISI
| PUBMED
16. Besag FMC, Dulac O, Alving J, Mullens EL. Long-term safety and efficacy of lamotrigine (Lamictal) in pediatric patients with epilepsy. Seizure. 1997;6:51-56.
FULL TEXT
|
ISI
| PUBMED
17. Faught E, Wilder BJ, Ramsay RE, et al. Topiramate placebo-controlled dose-ranging trial in refractory partial epilepsy using 200-, 400-, 600-mg daily dosages. Neurology. 1996;46:1684-1690.
FREE FULL TEXT
18. Privitera M, Fincham R, Penry J, et al. Topiramate placebo-controlled dose-ranging trial in refractory partial epilepsy using 600-, 800-, and 1,000-mg daily dosages. Neurology. 1996;46:1678-1683.
FREE FULL TEXT
19. Elterman R, Glauser TA, Wyllie E, Reife R, Wu S-C and the Topiramate YP Study Group. A double-blind, randomized trial of topiramate as adjunctive therapy for partial onset seizures in children. Neurology. 1999;52:1338-1344.
FREE FULL TEXT
20. Sachdeo RC, Reife RA, Lim P, Pledger G. Topiramate monotherapy for partial onset seizures. Epilepsia. 1997;38:294-300.
FULL TEXT
|
ISI
| PUBMED
21. Biton V, Montouris GD, Riviello JD, Reife R and the Topiramate YTC Study Group. Topiramate as add-on therapy in generalized seizures of non-focal origin. Ann Neurol. 1997;42:502-503.
22. Glauser TA. Topiramate. Epilepsia. 1999;40(suppl 5):S71-S80.
23. Post RM, Frye MA, Leverich GS, et al. The role of complex combination therapy in the treatment of refractory bipolar illness. CNS Spectrum. 1998;3:66-86.
24. Wheeler SD, Carrazana EJ. Topiramate treated cluster headaches. Neurology. 1999;53:234-236.
FREE FULL TEXT
25. Tatum IV WO, French JA, Faught E, Williams S. Postmarketing experience with topiramate and cognition [abstract]. Epilepsia. 1998;39:56.
26. Uthman BM, Rowan AJ, Ahmann PA, et al. Tiagabine for complex partial seizures: a randomized, add-on, dose-response trial. Arch Neurol. 1998;55:56-62.
FREE FULL TEXT
27. Sachdeo RC, Leroy RF, Krauss GL, et al. Tiagabine therapy for complex partial seizures. Arch Neurol. 1997;54:595-601.
FREE FULL TEXT
28. Schacter SC, Cahill WT, Wannamaker BB, Shu VS, Sommerville KW. Open-label dosage and tolerability study of tiagabine monotherapy in patients with refractory complex partial seizures. J Epilepsy. 1998;11:248-255.
FULL TEXT
|
ISI
29. Shorvon SD, van Rijckevorsel K, Lennox W, et al. Pooled efficacy and safety data of levetiracetam used as adjunctive therapy in patients with partial onset seizures [abstract]. Epilepsia. 1999;40(suppl 7):76.
30. Keppra [package insert]. Smyrna, Ga; UCB Pharmaceuticals Inc; 2000.
31. Klitgaard H, Matagne A, Govert J, Wulfert E. Evidence for a unique profile ofleviteracetam in rodent models of seizures and epilepsy. Eur J Pharmacol. 1998;353:191-206.
FULL TEXT
|
ISI
| PUBMED
32. Schacter SC. Oxcarbazepine: current status and clinical applications. Exp Opin Invest Drugs. 1999;8:1103-1112.
FULL TEXT
33. Tecoma ES. Oxcarbazepine. Epilepsia. 1999;40(suppl 5):S37-S46.
34. Leppik IE. Zonisamide. Epilepsia. 1999;40(suppl 5):S23-S29.
35. Henry TR, Leppik IE, Gumnit RJ, Jacobs M. Progressive myoclonus epilepsy treated with zonisamide. Neurology. 1988;38:928-931.
FREE FULL TEXT
36. Zonegran [package insert]. South San Francisco, Calif: Elan Pharmaceuticals Inc; 2000.
37. Ramsay RE, DeToledo J. Intravenous administration of fosphenytoin: options for the management of seizures. Neurology. 1996;46(6 suppl 1):S17-S19.
38. Uthman BM, Wilder BJ, Ramsay RE. Intramuscular use of fosphenytoin: an overview. Neurology. 1996;46(6 suppl 1):S24-S28
39. Mattson RH. Efficacy and adverse effects of established and new antiepileptic drugs. Epilepsia. 1995;36(suppl 2):S13-S26
40. Wilder BJ. The treatment of epilepsy: an overview of clinical practices. Neurology. 1995;45(suppl 2):S7-S11.
41. Brodie MJ, Dichter MA. Antiepileptic drugs. N Engl J Med. 1996;334:168-175.
FREE FULL TEXT
42. French J, Smith M, Faught E, et al. Practice advisory: the use of felbamate in the treatment of patients with intractable epilepsy. Epilepsia. 1999;40:803-808.
FULL TEXT
|
ISI
43. Snead OC, Hosey LC. Exacerbation of seizures in children by carbamazepine. N Engl J Med. 1985;313:916-921.
ABSTRACT
44. Tatum WO, Zachariah SB. Gabapentin treatment of seizures in acute intermittent porphyria. Neurology. 1995;45:1216-1217.
FREE FULL TEXT
THIS ARTICLE HAS BEEN CITED BY OTHER ARTICLES
Updates on the Treatment of Epilepsy in Women
Tatum et al.
Arch Intern Med 2004;164:137-145.
ABSTRACT
| FULL TEXT
|