Lee Limbird: "Always thirty seconds away from a changed life"
The phone has been persistently ringing in the office of the Associate Vice-Chancellor for Research at Vanderbilt Medical Center. Finally, Lee Limbird rushes in to take the pre-arranged call—a Department Chair at the Medical Center has been attempting to speak with her about the allocation of lab space. “Hi—I had to pee and they locked the restrooms on this floor,” she explains, as if apologizing to a close friend. And then, without pausing, “How can I help?”

This first encounter with Limbird is at odds with the staid, professional image that might be construed from a look through her ponderous CV: Phi Beta Kappa as an undergraduate; completion of her PhD in less than three years; authorship on over 130 peer-reviewed papers; three years as Chair of the NIH study section on pharmacology; numerous awards, grants, and honors; and multiple editorial assignments, including two stints as Editor-in-Chief (along with Joel Hardman) of Goodman and Gilman's The Pharmacological Basis of Therapeutics. Prior to overseeing the research initiatives of Vanderbilt Medical School, she had been Chair of the Department of Pharmacology.
But in talking with Lee Limbird, her route to the position of Associate Vice-Chancellor for Research at one of the country's leading medical centers appears to have been neither fueled by professional ambition nor even vaguely calculated. Sure, she is enthusiastic about the intellectual challenges—the “fun”—of biomedical research. But her unrestrained approach to science and scientists seems to stem foremost from an egalitarian sense for the difficulties, privileges, and obligations faced by all members and spheres of society. (She has driven the bus that carries detained Nashville youth on field trips, and she has spent nights, along with her own children, in Nashville homeless shelters and in the Mayan villages of Guatemala.) She mentors her students not only in the dynamics of receptor biology (and biologists), but also in the difficulties of balancing one's goals and commitments.
Interviews with people in the world of pharmacologyLee Limbird:“Always thirty seconds away from a changed life.”
“You're always thirty seconds away from a changed life.”
MI: Let's talk about your background. Did you come from a scientific family?
LL: No, not at all. Neither of my parents went to college, and it was very important to them that my brother and I go. College was a wonderful privilege—I still think of it as a privilege. My brother, Jay, was five years older than I, and that spacing was planned so that we could both go to college. My dad worked at the post office in Philadelphia. My mom—although it probably would have been beneficial for her to work, financially—stayed at home. So I was spoiled with lots of attention.
MI: And you went to the College of Wooster, which is a small, liberal arts school. Were you scientifically minded when you started out there?
LL: Probably not. I actually wanted to go to Cornell, but I was turned down. I had a little problem in high school, and that was keeping this little muscle [indicates mouth as chatter-box] working. So I spent a lot of time in the corridor, and I did not get good letters of recommendation. So I was not accepted at Cornell. And by that point—and I think it's part of the idealism that happens when you're seventeen—I was kind of burnt out with Philadelphia, and the only place that I had applied that wasn't local was the College of Wooster in Ohio, a college committed to independent study. The August before college matriculation—just imagine my parents, just put yourself in their shoes—the month before I should go to college, and they've saved all these years for this, right? I can't decide if I'm going to college or if I'm going into the Peace Corps. They're completely bonkers.
MI: So this was the 1960s, right? Even then you were on the vanguard, wanting to go into the Peace Corps!
LL: Which really irritated by parents, because their point was, “Even in our own back yard, we have more than enough grief and need for help. So why don't you stay home and ‘save the world' at your backdoor?” We went to visit Wooster the August before I was to enroll and I liked it, so I decided to go there.
MI: And then how did you get drawn to science?
LL: I don't know exactly how it happened. I think of one faculty member, one department, that made learning fun. Because it was a liberal arts college, there was a distribution of courses that you had to take. So I took Chemistry my first year, enjoyed the department, enjoyed the faculty, and just kept taking it. Now there were other things that I took on the side, also as distribution requirements, that were really exciting to me, like Art History. And the fact was that we had to do independent study at Wooster, so your whole fourth year was devoted to a senior project with one professor. And your third year was pretty much spent getting ready to do the fourth year project—learning how to use primary sources for research, learning how to write, etc. I think that I could see chemistry as a more natural discipline for research than art history, where you would be more describing than researching.
MI: Was being at a liberal arts school, with its emphasis on teaching—was that something that stuck with you?
LL: Oh absolutely. Initially I went to graduate school with the intent to teach at a small liberal arts college. I started at University of North Carolina—because Duke just seemed way too arrogant when I interviewed with them, and at that point, because I was a product of the sixties, that arrogance was a turn-off. But I quit after nine months and signed up for an MAT degree (master of arts in teaching at Duke). But they assigned me to some place where I'd have to live outside of Durham. Well, you don't tell a newlywed that she's going to go live two hours away from her new husband. So I declined to enter that MAT program and started working as a technician at Duke. To make a long story short, the research that I did, which was very independent and was based on the research that I did as an undergraduate (which was the thread that got me the job), was recognized by the University of North Carolina as my PhD dissertation. I had finished all my courses for the PhD—which normally takes two years—during my first nine months in graduate school there.
MI: And what was the research project that you were involved in at Duke?
LL: James Sidbury, who was a pediatric endocrinologist, had a research lab in which he provided space for Dr. Charles Roe, with whom I developed a method for quantifying the isoenzymes of creatine phosphokinase [CPK]. The hypothesis at that time was that the release into circulating blood of the heart isoenzyme (CPK-MB) could be used to indicate the occurrence and severity of a heart attack in patients for whom an analysis was otherwise difficult, either because they had had a previous MI, or because they were undergoing coronary bypass surgery. It turns out that the MB isoenzyme is not a very good parallel of infarct size, but there was a literature at the time based on data from dogs that it indeed could reflect infarct size. However, the existence of the MB isoenzyme in the splanchnic organs of dogs (but not in human beings) was a confounding issue. My research as a PhD student was very clinical, and some of the methods that I developed became routine methods at Duke.

MI: So despite a couple of major rearrangements in your course of study, you managed to finish you PhD in just three years?
LL: If it had been intentional it would have failed! [Laughs.] But my husband was done with his first year in Duke Medical School and was in clinical rotations, so he truly was not home. That's why I got through two years of coursework in one year. Because I just took courses to keep myself from going batty. We were living on a graduate student stipend and I was traveling up and down the road all the time from Durham to Chapel Hill, so I had neither time nor money to do anything else but take courses and study. We weren't allowed to live in graduate housing at UNC because I was the student and not my husband. At that time, housing was reserved for male graduate students and their spouses only. Isn't that amazing? It's a hoot now to even think they could get away with that. But I just accepted it.
MI: And then how did it happen that you stayed at Duke after finishing your PhD?
LL: Well, we went on a long camping and hiking trip around the country for three weeks to look at residency positions. Tom was very willing to look at places where I might work. So we looked at places where people were working on CPK isoenzymes, because I didn't really have any guidance, and I just thought that postdocs were supposed to work on the same subject on which they did their doctoral work. But I realized that I was just totally unenthusiastic about the prospect of doing more of the same. And so basically, on the trip home—you have more than a little time to think and talk when you're in a Volkswagen going from Seattle to Durham—I just decided that more work on CPK wasn't for me. And Duke had offered Tom an orthopedic surgery residency position, so I decided just to look for something there. I was offered a position as a Cardiology Fellow, in part because Andy Wallace, who was Chief of Cardiology, had been closely following my doctoral research. He had just hired Bob Lefkowitz as a young pup on the faculty from his residency at Mass General, and he thought I would enjoy working with Bob. And so I became Bob's first postdoc.

MI: Well, you make it sound like your early career just developed according to happenstance…
LL: That's true. I believe that I made my first intentional choice when I was twenty-seven. It makes my husband angry whenever I say this, because by that time we'd been married six years! But somewhere around when we were twenty-seven, it became clear that we weren't going to be able to have children. And I thought, “Oh my god. I might be getting out of bed and doing lab research every day for the rest of my life! I better get serious about it!” So I started to spend much more time thinking about the questions that needed to be asked in the field. Now, in retrospect, you might look at some of the things I did as a postdoc and say they were “straw men” questions, but one model of adenylyl cyclase when I started as a postdoc was that hormone effects occurred by allosteric modulation of the enzyme within a single molecule (as portrayed by a schematic in an early paper of Earl Sutherland). In my early studies, I showed the receptor was separable from cyclase. Rodbell and Gilman showed that G proteins were separate entities, acting between receptor and cyclase. I then showed that agonist occupancy stabilized receptor interaction with these separate GTP-binding proteins. Looking back now, some of my work seems pedantic. But it was nonetheless a great deal of fun!
MI: So do you think that it was just a matter of luck, up to that point at age twenty-seven, to have ended up in a position to ask those questions? Was there no career planning on your part before then?
LL: I was totally a ping-pong ball in a heated can. I was just very fortunate. There absolutely was no “career planning.” I just had one instance of good luck after another.
MI: But you certainly can't think that it was only luck! Honestly, weren't you some kind of a wiz, asking the right questions and taking in all that was going on in your research field?
LL: No, no. I was lucky. My whole way of thinking when I first became a postdoc was that I was just passing time until we had a family. My vision of my future was as a stay-at-home mother doing volunteer work.
MI: So you were really thinking very traditionally at that time, weren't you?
LL: Oh yes. This is why I never put two and two together—the fact that these guys were first author on my doctoral work, even though I worked very independently, and in some papers the first authors had no idea how to explain the approach or the data! The fact that no one offered career advice, the acceptance that we could not live in graduate housing at UNC—I was very naïve.
MI: You never looked on the challenge of becoming a successful woman in a traditionally male profession as a mission?
LL: I just think that I tuned into all of those issues later. Because I had been very lucky: I had a terrific professor in undergraduate school, Ted Williams—he had four daughters—who was all about creating opportunities, and it didn't matter whether they were for male or female students. Although as a PhD student I worked with people who didn't allow me to be first author, it didn't matter, because I didn't want to pursue that research area. And when I got into Bob's lab, his reason for hiring me had nothing to do with my background. I'm sure, if you asked him, he'd tell you he hired me because I was the wife of a surgical resident at Duke. And he knew that I would work nights and weekends because my husband wouldn't be home. Isn't that a hoot? And did that bother me? No! I couldn't have agreed more! It was fun to work with Bob, because he's a character—he's a raconteur par excellence. He's always having the time of his life—he just has fun. And the people in that lab also had fun, although I don't think if you were lazy that you'd enjoy being there because Bob was always wanting more and more and more data. But so did you, so it wasn't a conflict.
MI: So by luck you found yourself in a position to find out what science is about. And what else did you find out?
LL: Oh, I found out I loved it…I had a passion for doing experiments with my own hands. It's a real high to design an experiment that's discriminating—that's a lot of fun. I also loved it when I was first on the faculty, working with students and an occasional postdoc, because students have no biases. They take huge risks, and they don't care—they're just learning. And so I liked teaching, because I love to learn, and the only way to teach is to keep on learning. And for me, “teaching” means teaching people how to learn on their own, and eliminating the fear of failure—those are really the two elements of teaching. I do not enjoy taking a body of knowledge and passing it on. That, to me, is boring.
MI: Are you still able, in your current position, to pursue your passion for teaching and research?
LL: Oh I'm excited about a lot of things we're doing in the lab. For example, let me tell you one thing that's really cool: We have made some mice that are genetically modified with respect to the alpha-2a adrenergic receptor. First we just made a subtle mutation in the receptor, but it behaved sort of as a “functional knockout,” because the receptor didn't get expressed well. So then Brian Kobilka [Stanford University] made some knockout animals, and together our labs have collaborated with others to learn a lot about what this receptor subtype does: it centrally lowers blood pressure; it mediates sedation to agonists; it suppresses epileptogenesis—this is a surprise, but it's kind of exciting; we know from some behavioral studies that the receptor is probably protective against anxiety and depressive behaviors—that's new; and it mediates the suppression of pain reception.
So, Matt Wilson, one of my students, and then subsequently Chris Tan in my lab showed that if you make heterozygous animals, then they can't be sedated with alpha-2 agonists, but their blood pressure can still be lowered. This is mind-boggling, because to me it says: OK, to sedate, you've got to activate more than fifty percent of alpha-2a receptors. But to lower blood pressure, you only need to activate a minority of the receptors. So then you start to think: Ah, what's the side effect of alpha-2 agonists that's the most limiting in terms of clinical use? Sedation! Not a problem, if you're using alpha-2 agonists as preanasthetics, but sedation is not useful in antihypertensive treatment: CEOs cannot go to sleep—they'll be fired, right? And there's evidence both from nonhuman primate studies as well as from human clinical trials that alpha-2 agonists improve attentional focus, so they can be used in ADHD, but sedation is an unwanted side effect here, too. But just imagine now that you had a very inefficacious partial agonist at alpha-2a receptors; it might improve attentional focus, it might suppress epileptogenesis, but it wouldn't have the sedative side effects! So we're beginning studies to try to figure those things out.
I've just finished editing, with Joel Hardman, the upcoming Tenth Edition of Goodman and Gilman. This wasn't the most fun job that I've ever done, but I did learn pharmacology. And one of the things I learned reading the chapter (written by Jim McNamara) on anti-epileptic treatments was that there are a lot of anticonvulsants, and they help about 65% of patients, but there is nothing available to suppress epilepsy development. So I'm not saying that alpha-2a receptors suppress all epilepsy, but in a particular model, they suppress kindling behavior—we collaborated with Jim McNamara to demonstrate this in our genetically altered mice. And that model of plasticity has been considered a model for epilepsy. The possibility exists that there will be certain kinds of seizure events that can be suppressed by alpha-2 agonists and that if you could develop partial agonists you might find one that fortuitously suppresses epileptogenesis without causing sedation. Just like we recently identified a partial agonist at alpha-2 receptors that suppresses blood pressure but doesn't evoke sedation. So that's kind of cool.
MI: What are your administrative functions as Associate Vice-Chancellor for Research?
LL: My role is to articulate where different lines of research are going and how they will have a high impact. I link investigators together; I work with colleagues to develop Core laboratories to accelerate discoveries; I seek novel sources of new research resources; I just share in the fun of discovery! There are a lot of things out there that can be discovered. Certainly knowing the sequence of the human genome is going to change the slope of the rate of discovery. This is very exciting. In my own career, for example, I went from in vivo, to molecules, to in vivo again—and I did that over thirty-some years. Now, that cycle of research is going to occur much more rapidly for those investigators who follow a single line of inquiry. That's why it's so important for people to understand the vocabulary of molecules as well as the vocabulary of in vivo reflexes.
And that's why I think that pharmacology is a valuable discipline. Very, very, very exciting things are going on in pharmacology. I think sometimes people inside the discipline put a bell jar over certain subsets of the discipline and define those areas as real pharmacology. I understand the need for scholars of the discipline, people who fertilize the garden for the next generation. But when somebody thinks they're “protecting” the discipline—that can be the equivalent of putting it in a seal-a-meal bag, and after a while there's not enough oxygen for life. Pharmacology is not unique in this respect; there are some people who worry about what's real physiology and what's real this and what's real that. What's real biochemistry? I don't know. But I do know that some of the most exciting advances are likely to come from the disciplines that previously did not share vocabularies, like biology and physics, and biology and chemistry, and engineering and cell biology. And pharmacology must stay part of that mix!
People can be reluctant to get out of their comfort zone. That frustrates me. Instead of endorsing gene therapy, or stem cell research, there are investigators who argue about whether these areas are real pharmacology. But gene therapy is going to go nowhere until somebody figures out how to deal with the complexity of putting nucleotides in the body. So you administer a single “gene-based drug”—your body must make it into multiple metabolites: Does it always make it into the same ones? Are the metabolic consequences as predictable as for cytochrome P450s, or is metabolism really diverse? How heterogeneous are the products? What happens to them? Do they hurt you?
Who's asking those questions? Not enough people. There's no a priori reason that DNA delivered in a liposome will be the same as DNA delivered in phosphate-buffered saline or within an adenoviral construct. I mean, who's asking? If it were a small molecule, 300 MW, somebody would be asking. But because it's DNA, or other novel structures, not enough people are asking, because asking about these molecules may not be “real” pharmacology. That's kind of upsetting.
MI: So in addition to pursuing new technologies to answer your own research questions, you concern yourself with the ways that research is defined for the community. Is that sometimes a tough balancing act?
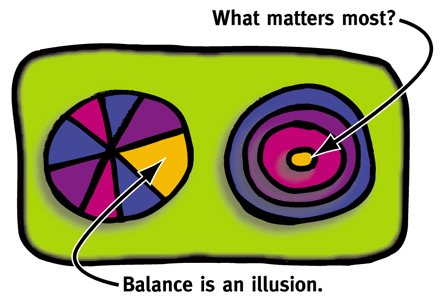
LL: You know, balance is an illusion. Students will sometimes ask about living in balance. And I draw them this diagram [see inset.] Some people think that this [drawing a pie diagram] is balance, but this is not balance; this is about giving exercise as much importance as time with family and time for experiments—or time for unimportant stuff. To me it's more important to know what's important to you [drawing a series of concentric rings], and if all else collapses in your life, then there's your center and that's where you focus. And if all is well in your life, then you can enjoy all of the rings. But if all is not well in the world, you have to know where your center is. What matters most? To a certain extent, the center is constant, but to a certain extent, it's a reflection of the seasons of our lives. We are not the same in our twenties as in our thirties or in our forties and our fifties; and it's a good thing. Because society needs the blinded passion and doggedness of people in their twenties and thirties. But society also needs the wisdom of people who have seen a lot pass by them and who have learned not to fret about everything. But if you were that objective about things when you were younger, you wouldn't have committed yourself with tireless passion—and society would not have been the beneficiary of as many discoveries from these young, energetic, highly focused investigators. It's nice that there are different seasons. It will be even better when the research enterprise—in the academic as well as in the for-profit world—learns to take best advantage of scientists in these varying seasons of their lives.
- © American Society for Pharmacology and Experimental Theraputics 2001



